Plasma concentrations of 5-fluorouracil and F-beta-alanine following oral administration of S-1, a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5-fluorouracil
- PMID: 12942110
- PMCID: PMC2394492
- DOI: 10.1038/sj.bjc.6601224
Plasma concentrations of 5-fluorouracil and F-beta-alanine following oral administration of S-1, a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5-fluorouracil
Abstract
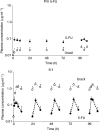
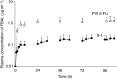
The pharmacokinetics and pharmacodynamics of oral S-1, a dihydropyrimidine dehydrogenase (DPD) inhibitory fluoropyrimidine, were compared with those of protracted venous infusion (PVI) of 5-fluorouracil (5-FU). In all, 10 patients with gastric cancer received PVI of 5-FU at a dose of 250 mg m(-2) day(-1) for 5 days. After a washout period of 9 days, the patients received two divided doses daily for 28 days. S-1 was administered orally at about 0900 and 1900 hours. The daily dose of S-1 in terms of tegafur was 80 mg day(-1) in patients with a body surface area (BSA) of <1.25 m(2), 100 mg day(-1) in those with a BSA of >or=1.25 m(2) to <1.5 m(2), and 120 mg day(-1) in those with a BSA of >or=1.5 m(2). Plasma concentrations of 5-FU and F-beta-alanine (FBAL) were measured for pharmacokinetic analysis, and the plasma uracil concentration was monitored as a surrogate marker of DPD inhibition (pharmacodynamic analysis) in the same patients on days 1-5 of PVI of 5-FU and on days 1-5 of oral S-1. The area under the curve (AUC(0-10 h)) of 5-FU on day 5 was 728+/-113 ng h ml(-1) for PVI of 5-FU and 1364+/-374 ng h ml(-1) for S-1. The median 5-FU PVI : S-1 ratio of the AUC(0-10 h) of 5-FU was 1.9. The AUC(0-10 h) of FBAL on day 5 of PVI of 5-FU was 9465+/-3225 ng h ml(-1), AUC(0-10 h), as compared with 1725+/-605 ng h ml(-1) on day 5 of S-1 treatment. The AUC(0-10 h) of uracil on day 5 was 252+/-60 ng h ml(-1) with PVI of 5-FU and 12 582+/-3060 ng h ml(-1) with S-1. The AUC(0-10 h) of FBAL was markedly lower and plasma uracil concentrations were significantly higher for S-1 than for PVI of 5-FU, clearly demonstrating the effect of DPD inhibition.
Figures
Similar articles
-
[Plasma concentrations of 5-fluorouracil and F-beta-alanine following oral administration of S-1, a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5-fluorouracil].Gan To Kagaku Ryoho. 2006 Jun;33 Suppl 1:37-41. Gan To Kagaku Ryoho. 2006. PMID: 16897970 Japanese.
-
Comparative pharmacokinetic study of continuous venous infusion fluorouracil and oral fluorouracil with eniluracil in patients with advanced solid tumors.J Clin Oncol. 2002 Mar 15;20(6):1683-91. doi: 10.1200/JCO.2002.20.6.1683. J Clin Oncol. 2002. PMID: 11896120 Clinical Trial.
-
Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2'-tetrahydrofuryl)-5-fluorouracil.Clin Cancer Res. 1998 Sep;4(9):2085-8. Clin Cancer Res. 1998. PMID: 9748123
-
Oral DPD-inhibitory fluoropyrimidine drugs.Oncology (Williston Park). 2000 Oct;14(10 Suppl 9):19-23. Oncology (Williston Park). 2000. PMID: 11098485 Review.
-
The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors.Anticancer Drugs. 2004 Feb;15(2):85-106. doi: 10.1097/00001813-200402000-00001. Anticancer Drugs. 2004. PMID: 15075664 Review.
Cited by
-
5-Fluorouracil cardiotoxicity induced by alpha-fluoro-beta-alanine.Int J Clin Oncol. 2005 Dec;10(6):441-3. doi: 10.1007/s10147-005-0516-7. Int J Clin Oncol. 2005. PMID: 16369751
-
A Study of the S-1 or Capecitabine as First-line Regimen in Patients with Metastatic Colorectal Cancer: A Real World Study.J Cancer. 2020 Jan 20;11(7):1839-1845. doi: 10.7150/jca.36929. eCollection 2020. J Cancer. 2020. PMID: 32194795 Free PMC article.
-
A phase I study of sorafenib in combination with S-1 plus cisplatin in patients with advanced gastric cancer.Gastric Cancer. 2014 Jan;17(1):161-72. doi: 10.1007/s10120-013-0247-9. Epub 2013 Mar 27. Gastric Cancer. 2014. PMID: 23532594 Clinical Trial.
-
UFT (tegafur and uracil) as postoperative adjuvant chemotherapy for solid tumors (carcinoma of the lung, stomach, colon/rectum, and breast): clinical evidence, mechanism of action, and future direction.Surg Today. 2007;37(11):923-43. doi: 10.1007/s00595-007-3578-5. Epub 2007 Oct 25. Surg Today. 2007. PMID: 17952521 Review.
-
Phase I/II study of oxaliplatin with oral S-1 as first-line therapy for patients with metastatic colorectal cancer.Br J Cancer. 2008 Mar 25;98(6):1034-8. doi: 10.1038/sj.bjc.6604271. Epub 2008 Mar 4. Br J Cancer. 2008. PMID: 18319719 Free PMC article. Clinical Trial.
References
-
- Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, Behr J, Gordon RJ, Osterwalder B, Griffin T (1998) Preliminary studies of a novel fluoropyrimidine carbamate: capecitabine. J Clin Oncol 16: 1795–1802 - PubMed
-
- Chollet P, Schoffski P, Weigang-Kohler K, Schellens JHM, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau P (2003) Phase II trial with S-1 in chemotherapy-naïve patients with gastric cancer. A trial performed by the EORTC early clinical studies group (ECSG). Eur J Cancer 39: 1264–1270 - PubMed
-
- Cohen SJ, Leichman CG, Yeslow G, Beard M, Proefrock A, Roedig B, Damle B, Letrent SP, DeCillis AP, Meropol NJ (2002) Phase I and pharmacokinetic study of once daily oral administration of S-1 in patients with advanced cancer. Clin Cancer Res 8: 2116–2122 - PubMed
-
- Diasio RB (1998) The role of dihydropyrimidine dehydrogenase (DPD) modulation in 5-FU pharmacology. Oncology 12: 23–27 - PubMed
-
- Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R (2001) Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19: 2282–2292 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

