The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media
- PMID: 12943368
- PMCID: PMC3202714
- DOI: 10.1007/s10162-002-3015-9
The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media
Abstract
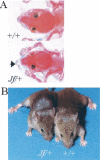
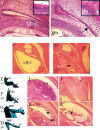
Otitis media is the most common cause of hearing impairment in children and is primarily characterized by inflammation of the middle ear mucosa. Yet nothing is known of the underlying genetic pathways predisposing to otitis media in the human population. Increasingly, large-scale mouse mutagenesis programs have undertaken systematic and genome-wide efforts to recover large numbers of novel mutations affecting a diverse array of phenotypic areas involved with genetic disease including deafness. As part of the UK mutagenesis program, we have identified a novel deaf mouse mutant, Jeff (Jf). Jeff maps to the distal region of mouse chromosome 17 and presents with fluid and pus in the middle ear cavity. Jeff mutants are 21% smaller than wild-type littermates, have a mild craniofacial abnormality, and have elevated hearing thresholds. Middle ear epithelia of Jeff mice show evidence of a chronic proliferative otitis media. The Jeff mutant should prove valuable in elucidating the underlying genetic pathways predisposing to otitis media.
Figures




References
-
- Ball SS, Prazma J, Dais CG, Triana RJ, Pillsbury HC. Role of tumor necrosis factor and interleukin-1 in endotoxin-induced middle ear effusions. Ann. Otol. Rhinol. Laryngol. 1997;106:633–639. - PubMed
-
- Berry CL, Vogler C, Galvin NJ, Birkenmeier EH, Sly WS. Pathology of the ear in murine mucopolysaccharidosis type VII. Morphological correlates of hearing loss. Lab. Invest. 1994;71:438–445. - PubMed
-
- Brown SDM, Steel KP (2002) DFN genes. In: Creighton TE (ed) Encyclopedia of Molecular Medicine. John Wiley & Sons, New York, pp 1035–1038
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

