Distribution of gentamicin in the guinea pig inner ear after local or systemic application
- PMID: 12943372
- PMCID: PMC3202710
- DOI: 10.1007/s10162-002-2036-8
Distribution of gentamicin in the guinea pig inner ear after local or systemic application
Abstract
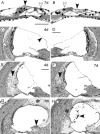
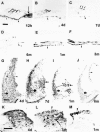
Uptake and retention of gentamicin by cells in the guinea pig inner ear after a single peritoneal injection or local application on the round window were investigated using immunocytochemistry to localize the drug. The cells that accumulated the drug under the two conditions were the same, but staining for the drug was more intense and was often accompanied by widespread cochlear degeneration following local application. Soon after drug administration by either route, there was diffuse staining for the drug throughout all tissue within the labyrinth, including bone. At later times when distinct cell staining became evident, virtually all cell types were found to be positive, with several cell types staining more darkly for the drug than hair cells, indicating that hair cells were not the most avid in accumulating gentamicin. The infracuticular portion of auditory and vestibular hair cells as well as type III fibrocytes of the spiral ligament were positively stained in almost all cases and these sites were found to be positive for as long as six months post administration. In animals with loss of the organ of Corti, there was unusually intense staining for gentamicin in root cells of the spiral ligament, in marginal cells of the stria vascularis, and in cells of the spiral limbus. Dark staining of surviving cells in cases with overt tissue destruction suggests that variability in the extent of damage caused by the drug was determined more by the degree of its local uptake than by differences in animals' capacities to metabolize the drug systemically. The present results show that gentamicin may damage or destroy all cochlear cells following a single round window application. The findings broaden the scope of our knowledge of cochlear gentamicin uptake and damage and have implications for treatment of patients with vestibular disorders by infusion of aminoglycosides into the middle ear, as well as implications for prospects of rehabilitating patients that have been deafened by aminoglycosides.
Figures





References
-
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J. Histochem. Cytochem. 1992;40:1457–1463. - PubMed
-
- Aran JM, Erre JP, Lima da Costa D, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Ann. N. Y. Acad. Sci. 1999;884:60–68. - PubMed
-
- Balogh K, Hiraide F, Ishi D. Distribution of radioactive dihydrostreptomycin in the cochlea—an autoradiographic study. Ann. Otol. (St. Louis) 1970;79:641–652. - PubMed
-
- Blakley BW. Update on intratympanic gentamicin for Meniere's disease. Laryngoscope. 2000;110:236–240. - PubMed
-
- Brummett RE, Traynor J, Brown R, Himes D. Cochlear damage resulting from kanamycin and furosemide. Acta Otolaryngol. 1975;80:86–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

