Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio)
- PMID: 12943374
- PMCID: PMC3202713
- DOI: 10.1007/s10162-002-3022-x
Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio)
Abstract
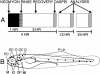
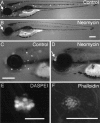
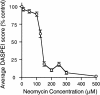
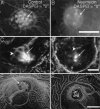
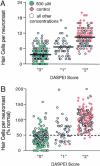
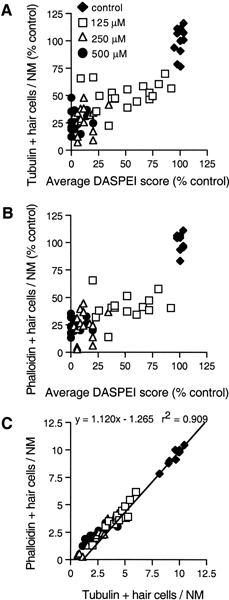
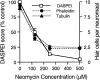
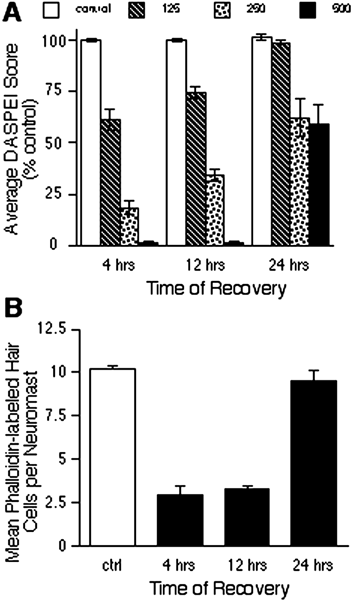
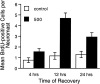
Mechanoreceptive hair cells are extremely sensitive to aminoglycoside antibiotics, including neomycin. Hair cell survival was assessed in larval wild-type zebrafish lateral line neuromasts 4 h after initial exposure to a range of neomycin concentrations for 1 h. Each of the lateral line neuromasts was scored in live fish for the presence or absence of hair cells using the fluorescent vital dye DASPEI to selectively label hair cells. All neuromasts were devoid of DASPEI-labeled hair cells 4 h after 500 microM neomycin exposure. Vital DASPEI staining was proportional to the number of hair cells per neuromast identified in fixed larvae using immunocytochemistry for acetylated tubulin and phalloidin labeling. The time course of hair cell regeneration in the lateral line neuromasts was also analyzed following neomycin-induced damage. Regenerated hair cells were first observed using live DASPEI staining 12 and 24 h following neomycin treatment. The potential role of proliferation in regenerating hair cells was analyzed. A 1 h pulse-fix protocol using bromodeoxyuridine (BrdU) incorporation was used to identify S-phase cells in neuromasts. BrdU incorporation in neomycin-damaged neuromasts did not differ from control neuromasts 4 h after drug exposure but was dramatically upregulated after 12 h. The proliferative cells identified during a 1 h period at 12 h after neomycin treatment were able to give rise to new hair cells by 24-48 h after drug treatment. The results presented here provide a standardized preparation for studying and identifying genes that influence vertebrate hair cell death, survival, and regeneration following ototoxic insults.
Figures




Similar articles
-
Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line.J Neurosci. 2008 Feb 27;28(9):2261-73. doi: 10.1523/JNEUROSCI.4372-07.2008. J Neurosci. 2008. PMID: 18305259 Free PMC article.
-
Neomycin damage and regeneration of hair cells in both mechanoreceptor and electroreceptor lateral line organs of the larval Siberian sturgeon (Acipenser baerii).J Comp Neurol. 2016 May 1;524(7):1443-56. doi: 10.1002/cne.23918. Epub 2015 Nov 23. J Comp Neurol. 2016. PMID: 26502298
-
Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio).Hear Res. 2003 Dec;186(1-2):47-56. doi: 10.1016/s0378-5955(03)00259-4. Hear Res. 2003. PMID: 14644458
-
There and back again: development and regeneration of the zebrafish lateral line system.Wiley Interdiscip Rev Dev Biol. 2015 Jan-Feb;4(1):1-16. doi: 10.1002/wdev.160. Epub 2014 Oct 20. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25330982 Free PMC article. Review.
-
Chemical Ototoxicity of the Fish Inner Ear and Lateral Line.Adv Exp Med Biol. 2016;877:419-37. doi: 10.1007/978-3-319-21059-9_18. Adv Exp Med Biol. 2016. PMID: 26515324 Review.
Cited by
-
Transdifferentiation is temporally uncoupled from progenitor pool expansion during hair cell regeneration in the zebrafish inner ear.Development. 2024 Aug 1;151(15):dev202944. doi: 10.1242/dev.202944. Epub 2024 Aug 13. Development. 2024. PMID: 39045613 Free PMC article.
-
Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells.PLoS One. 2012;7(2):e29727. doi: 10.1371/journal.pone.0029727. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359538 Free PMC article.
-
Sensory hair cell death and regeneration in fishes.Front Cell Neurosci. 2015 Apr 21;9:131. doi: 10.3389/fncel.2015.00131. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25954154 Free PMC article. Review.
-
A convergent and essential interneuron pathway for Mauthner-cell-mediated escapes.Curr Biol. 2015 Jun 1;25(11):1526-34. doi: 10.1016/j.cub.2015.04.025. Epub 2015 May 7. Curr Biol. 2015. PMID: 25959971 Free PMC article.
-
Alcohol-induced morphological deficits in the development of octavolateral organs of the zebrafish (Danio rerio).Zebrafish. 2013 Mar;10(1):52-61. doi: 10.1089/zeb.2012.0830. Epub 2013 Mar 5. Zebrafish. 2013. PMID: 23461415 Free PMC article.
References
-
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann. N.Y. Acad. Sci. 1996;781:59–70. - PubMed
-
- Carey JP, Fuchs AF, Rubel EW. Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J. Neurophysiol. 1996;76:3301–3312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

