Enlargement and contracture of C2-ceramide channels
- PMID: 12944273
- PMCID: PMC1303332
- DOI: 10.1016/S0006-3495(03)74588-3
Enlargement and contracture of C2-ceramide channels
Abstract
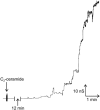
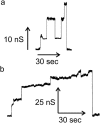
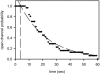
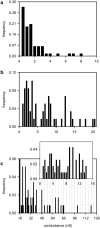
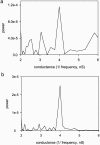
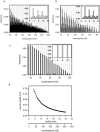
Ceramides are known to play a major regulatory role in apoptosis by inducing cytochrome c release from mitochondria. We have previously reported that ceramide, but not dihydroceramide, forms large and stable channels in phospholipid membranes and outer membranes of isolated mitochondria. C(2)-ceramide channel formation is characterized by conductance increments ranging from <1 to >200 nS. These conductance increments often represent the enlargement and contracture of channels rather than the opening and closure of independent channels. Enlargement is supported by the observation that many small conductance increments can lead to a large decrement. Also the initial conductances favor cations, but this selectivity drops dramatically with increasing total conductance. La(+3) causes rapid ceramide channel disassembly in a manner indicative of large conducting structures. These channels have a propensity to contract by a defined size (often multiples of 4 nS) indicating the formation of cylindrical channels with preferred diameters rather than a continuum of sizes. The results are consistent with ceramides forming barrel-stave channels whose size can change by loss or insertion of multiple ceramide columns.
Figures





References
-
- Akutsu, H., and J. Seelig. 1981. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 20:7366–7373. - PubMed
-
- Amarante-Mendes, G. P., C. Naekyung Kim, L. Liu, Y. Huang, C. L. Perkins, D. R. Green, and K. Bhalla. 1998. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood. 91:1700–1705. - PubMed
-
- Antonsson, B., S. Montessuit, B. Sanchez, and J. C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615–11623. - PubMed
-
- Ariga, T., W. D. Jarvis, and R. K. Yu. 1998. Role of sphingolipid-mediated cell death in neurodegenerative diseases. J. Lipid Res. 39:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

