Lipid bilayer vesicle fusion: intermediates captured by high-speed microfluorescence spectroscopy
- PMID: 12944275
- PMCID: PMC1303334
- DOI: 10.1016/S0006-3495(03)74590-1
Lipid bilayer vesicle fusion: intermediates captured by high-speed microfluorescence spectroscopy
Abstract
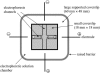
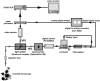
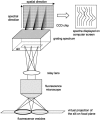
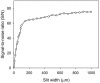
The fusion of lipid bilayers can be visualized under the fluorescence microscope, but the process is very fast and requires special techniques for its study. It is reported here that vesicle fusion is susceptible to analysis by microspectrofluorometry and that for the first time, the entire fusion process has been captured. In the case of giant (>10- micro m diameter) bilayer vesicles having a high density of opposite charge, fusion proceeds through stages of adhesion, flattening, hemifusion, elimination of the intervening septum, and uptake of excess membrane to generate a spherical product very rapidly. These investigations became possible with a fluorescence microscope that was modified for recording of images simultaneously with the collection of fluorescence emission spectra from many (>100) positions along the fusion axis. Positively-charged vesicles, composed of O-ethylphosphatidylcholine and dioleoylphosphatidylcholine, were labeled with a carbocyanine fluorophore. Negatively-charged vesicles, composed of dioleoylphosphatidylglycerol and dioleoylphosphatidylcholine, were labeled with a rhodamine fluorophore that is a resonance energy transfer acceptor from the carbocyanine fluorophore. An electrophoretic chamber allowed selection of pairs of vesicles to be brought into contact and examined. Spectral changes along the axis of fusion were captured at high speed (a few ms/frame) by operating a sensitive digital camera in the virtual-chip mode, a software/hardware procedure that permits rapid readout of selected regions of interest and by pixel binning along the spectral direction. Simultaneously, color images were collected at video rates (30 frame/s). Comparison of the spectra and images revealed that vesicle fusion typically passes through a hemifusion stage and that the time from vesicle contact to fusion is <10 ms. Fluorescence spectra are well suited to rapid collection in the virtual-chip mode because spectra (in contrast to images) are accurately characterized with a relatively small number of points and interfering signals can be removed by judicious choice of barrier filters. The system should be especially well-suited to phenomena exhibiting rapid fluorescence change along an axis; under optimal conditions, it is possible to obtain sets of spectra (wavelength range of approximately 150 nm) at >100 positions along a line at rates >1000 frames/s with a spectral resolution of approximately 10 nm and spatial resolution at the limit of the light microscope ( approximately 0.2 micro m).
Figures









Similar articles
-
Effects on interactions of oppositely charged phospholipid vesicles of covalent attachment of polyethylene glycol oligomers to their surfaces: adhesion, hemifusion, full fusion and "endocytosis".J Membr Biol. 2008 Jan;221(2):97-106. doi: 10.1007/s00232-007-9089-x. Epub 2008 Jan 18. J Membr Biol. 2008. PMID: 18202882
-
Cholesterol stabilizes hemifused phospholipid bilayer vesicles.Biochim Biophys Acta. 2001 Apr 2;1511(2):264-70. doi: 10.1016/s0005-2736(01)00283-8. Biochim Biophys Acta. 2001. PMID: 11286969
-
Directly observed membrane fusion between oppositely charged phospholipid bilayers.J Membr Biol. 1999 Jul 1;170(1):27-38. doi: 10.1007/s002329900535. J Membr Biol. 1999. PMID: 10398758
-
Indirect evidence of submicroscopic pores in giant unilamellar [correction of unilamelar] vesicles.Biochim Biophys Acta. 2005 Aug 5;1724(3):281-7. doi: 10.1016/j.bbagen.2005.04.028. Biochim Biophys Acta. 2005. PMID: 15978732 Review.
-
Selected tools to visualize membrane interactions.Eur Biophys J. 2021 Mar;50(2):211-222. doi: 10.1007/s00249-021-01516-6. Epub 2021 Mar 31. Eur Biophys J. 2021. PMID: 33787948 Free PMC article. Review.
Cited by
-
Asymmetric Lipid Transfer between Zwitterionic Vesicles by Nanoviscosity Measurements.Nanomaterials (Basel). 2021 Apr 22;11(5):1087. doi: 10.3390/nano11051087. Nanomaterials (Basel). 2021. PMID: 33922325 Free PMC article.
-
Visualization of SNARE-Mediated Hemifusion between Giant Unilamellar Vesicles Arrested by Myricetin.Front Mol Neurosci. 2017 Mar 31;10:93. doi: 10.3389/fnmol.2017.00093. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28408867 Free PMC article.
-
Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection.J Am Chem Soc. 2011 Mar 23;133(11):4132-9. doi: 10.1021/ja111110e. Epub 2011 Feb 23. J Am Chem Soc. 2011. PMID: 21344925 Free PMC article.
-
A modular platform for one-step assembly of multi-component membrane systems by fusion of charged proteoliposomes.Nat Commun. 2016 Oct 6;7:13025. doi: 10.1038/ncomms13025. Nat Commun. 2016. PMID: 27708275 Free PMC article.
-
Direct visualization of large and protein-free hemifusion diaphragms.Biophys J. 2010 Apr 7;98(7):1192-9. doi: 10.1016/j.bpj.2009.11.042. Biophys J. 2010. PMID: 20371318 Free PMC article.
References
-
- Angelova, M. I., and D. S. Dimitrov. 1986. Liposome electroformation. Faraday Discuss. Chem. Soc. 81:303–311.
-
- Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Prog. Coll. Polym. 89:127–131.
-
- Chanturiya, A., P. Scaria, and M. C. Woodle. 2000. The role of membrane lateral tension in calcium-induced membrane fusion. J. Membr. Biol. 176:67–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

