Transport of nucleosome core particles in semidilute DNA solutions
- PMID: 12944295
- PMCID: PMC1303354
- DOI: 10.1016/S0006-3495(03)74610-4
Transport of nucleosome core particles in semidilute DNA solutions
Abstract
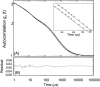
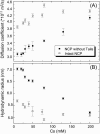
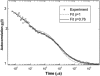
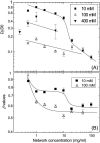
We studied the diffusion of native and trypsinized nucleosome core particles (NCPs), in aqueous solution and in concentrated DNA solutions (0.25-100 mg/ml) using fluorescence correlation spectroscopy (FCS). The highest DNA concentrations studied mimic the DNA density inside the cell nucleus. The diffusion coefficient of freely diffusing NCPs depends on the presence or absence of histone tails and is affected by the salt concentration due to the relaxation effect of counterions. NCPs placed in a network of long DNA molecules (30-50 kbp) reveal anomalous diffusion. We demonstrate that NCPs diffusion is in agreement with known particle transport in entangled macromolecular solutions as long as the histone tails are folded onto the particles. In contrast, when these tails are unfolded, the reversible adsorption of NCPs onto the DNA network has to be taken into account. This is confirmed by the fact that removal of the tails leads to reduction of the interaction between NCPs and the DNA network. The findings suggest that histone tail bridging plays an important role in chromatin dynamics.
Figures

References
-
- Ajdari, A. 1995. Transport by active filaments. Europhys. Lett. 31:69–74.
-
- Ausio, J., and K. V. Van Holdes. 1989. Use of selectively trypsinized nucleosome core particles to analyse the role of histone tails in the stabilisation of the nucleosome. J. Mol. Biol. 206:451–463. - PubMed
-
- Busch, N. A., T. Kim, and V. A. Bloomfield. 2000. Tracer diffusion of proteins in DNA solutions. 2 Green fluorescent protein in crowded solutions. Macromolecules. 33:5932–5937.
-
- Elson, E., and D. Madge. 1974. Fluorescence correlation spectroscopy- I. Conceptual basis and theory. Biopolymers. 13:1–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

