Mechanism of fluorescence and conformational changes of the sarcoplasmic calcium binding protein of the sand worm Nereis diversicolor upon Ca2+ or Mg2+ binding
- PMID: 12944301
- PMCID: PMC1303360
- DOI: 10.1016/S0006-3495(03)74616-5
Mechanism of fluorescence and conformational changes of the sarcoplasmic calcium binding protein of the sand worm Nereis diversicolor upon Ca2+ or Mg2+ binding
Abstract
The calcium-binding protein isolated from the sarcoplasm of the muscles of the sand worm Nereis diversicolor has four EF-hands and three active binding sites for Ca(2+) or Mg(2+). Nereis diversicolor sarcoplasmic calcium-binding protein contains three tryptophan residues at positions 4, 57, and 170, respectively. The Wt protein shows a very limited fluorescence increase upon binding of Ca(2+) or Mg(2+). Single-tryptophan-containing mutants were produced and purified. The fluorescence titrations of these mutants show a limited decrease of the affinity for calcium, but no alterations of the cooperativity. Upon adding calcium, Trp170 shows a strong fluorescence increase, Trp57 an extensive fluorescence decrease, and Trp4 shows no fluorescence change. Therefore mutant W4F/W170F is ideally suited to analyze the fluorescence titrations and to study the binding mechanism. Mutations of the calcium ligands at the z-position in the three binding sites show no effect at site I and a total loss of cooperativity at sites III and IV. The quenching of Trp57 upon calcium binding is dependent on the presence of arginine R25, but this residue is not just a simple dynamic quencher. The role of the salt bridge R25-D58 is also investigated.
Figures

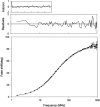
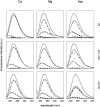
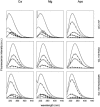

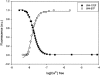
References
-
- Beechem, J. M., J. R. Knutson, J. B. A. Ross, B. W. Turner, and L. Brand. 1983. Global resolution of heterogeneous decay by phase modulation fluorometry: mixtures and proteins. Biochemistry. 22:6054–6058.
-
- Bevington, P. R. 1969. Gradient-expansion algorithm. In Data Reduction and Error Analyses for the Physical Sciences. McGraw-Hill, New York. pp.235–240.
-
- Burstein, E. A. 1983. The intrinsic luminescence of proteins is a method for studies of the fast structural dynamics. Mol. Biol. 17:455–467. - PubMed
-
- Burstein, E. A., and V. I. Emelyanenko. 1996. Log-normal description of fluorescence spectra of organic fluorophores. Photochem. Photobiol. 64:316–320.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

