Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter. A specific role for sialic acid residues
- PMID: 12944413
- PMCID: PMC3042025
- DOI: 10.1074/jbc.M306360200
Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter. A specific role for sialic acid residues
Retraction in
-
Withdrawal: Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter: a specific role for sialic acid residues.J Biol Chem. 2019 Jun 14;294(24):9657. doi: 10.1074/jbc.W119.009466. J Biol Chem. 2019. PMID: 31201245 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern: Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter: A specific role for sialic acid residues.J Biol Chem. 2019 Mar 29;294(13):5210. doi: 10.1074/jbc.EC119.008297. J Biol Chem. 2019. PMID: 30926758 Free PMC article. No abstract available.
Abstract
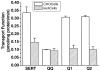
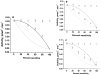
The serotonin transporter (SERT) is an oligomeric glycoprotein with two sialic acid residues on each of two complex oligosaccharide molecules. In this study, we investigated the contribution of N-glycosyl modification to the structure and function of SERT in two model systems: wild-type SERT expressed in sialic acid-defective Lec4 Chinese hamster ovary (CHO) cells and a mutant form (after site-directed mutagenesis of Asn-208 and Asn-217 to Gln) of SERT, QQ, expressed in parental CHO cells. In both systems, SERT monomers required modification with both complex oligosaccharide residues to associate with each other and to function in homo-oligomeric forms. However, defects in sialylated N-glycans did not alter surface expression of the SERT protein. Furthermore, in heterologous (CHO and Lec4 cells) and endogenous (placental choriocarcinoma JAR cells) expression systems, we tested whether glycosyl modification also manipulates the hetero-oligomeric interactions of SERT, specifically with myosin IIA. SERT is phosphorylated by cGMP-dependent protein kinase G through interactions with anchoring proteins, and myosin is a protein kinase G-anchoring protein. A physical interaction between myosin and SERT was apparent; however, defects in sialylated N-glycans impaired association of SERT with myosin as well as the stimulation of the serotonin uptake function in the cGMP-dependent pathway. We propose that sialylated N-glycans provide a favorable conformation to SERT that allows the transporter to function most efficiently via its protein-protein interactions.
Figures
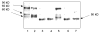




Similar articles
-
Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation.Mol Pharmacol. 2004 Jun;65(6):1462-74. doi: 10.1124/mol.65.6.1462. Mol Pharmacol. 2004. PMID: 15155839
-
cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake.Mol Brain. 2009 Aug 5;2:26. doi: 10.1186/1756-6606-2-26. Mol Brain. 2009. PMID: 19656393 Free PMC article.
-
Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters.J Neurochem. 2003 Jun;85(6):1513-20. doi: 10.1046/j.1471-4159.2003.01793.x. J Neurochem. 2003. PMID: 12787070 Free PMC article.
-
Molecular physiology of norepinephrine and serotonin transporters.J Exp Biol. 1994 Nov;196:263-81. doi: 10.1242/jeb.196.1.263. J Exp Biol. 1994. PMID: 7823027 Review.
-
Structure, function and regulation of the 5-hydroxytryptamine (serotonin) transporter.Biochem Soc Trans. 2001 Nov;29(Pt 6):728-32. doi: 10.1042/0300-5127:0290728. Biochem Soc Trans. 2001. PMID: 11709064 Review.
Cited by
-
The role of ERp44 in maturation of serotonin transporter protein.J Biol Chem. 2012 May 18;287(21):17801-17811. doi: 10.1074/jbc.M112.345058. Epub 2012 Mar 26. J Biol Chem. 2012. Retraction in: J Biol Chem. 2019 Jan 4;294(1):70. doi: 10.1074/jbc.W118.007073. PMID: 22451649 Free PMC article. Retracted.
-
The cellular distribution of serotonin transporter is impeded on serotonin-altered vimentin network.PLoS One. 2009;4(3):e4730. doi: 10.1371/journal.pone.0004730. Epub 2009 Mar 9. PLoS One. 2009. Retraction in: PLoS One. 2019 Feb 1;14(2):e0211966. doi: 10.1371/journal.pone.0211966. PMID: 19270731 Free PMC article. Retracted.
-
Regulatory mechanism of CCN2 production by serotonin (5-HT) via 5-HT2A and 5-HT2B receptors in chondrocytes.PLoS One. 2017 Nov 16;12(11):e0188014. doi: 10.1371/journal.pone.0188014. eCollection 2017. PLoS One. 2017. PMID: 29145495 Free PMC article.
-
At diabetes-like concentration, glucose down-regulates the placental serotonin transport system in a cell-cycle-dependent manner.J Neurochem. 2007 May;101(4):937-48. doi: 10.1111/j.1471-4159.2007.04469.x. Epub 2007 Mar 12. J Neurochem. 2007. Retraction in: J Neurochem. 2020 Apr;153(1):138. doi: 10.1111/jnc.14931. PMID: 17355243 Free PMC article. Retracted.
-
Effects of N-linked glycosylation on the creatine transporter.Biochem J. 2006 Jan 15;393(Pt 2):459-69. doi: 10.1042/BJ20050857. Biochem J. 2006. PMID: 16167890 Free PMC article.
References
-
- Blakely R, Berson H, Fremeau R, Jr, Caron M, Peek M, Priace H, Bradley C. Nature. 1991;354:66–70. - PubMed
-
- Hoffman B, Mezey E, Browstein M. Science. 1991;254:579–580. - PubMed
-
- Uhl G. Trends Neurosci. 1992;15:265–268. - PubMed
-
- Kanner B. J Exp Biol. 1994;196:237–249. - PubMed
-
- Amara S, Kuhar M. Annu Rev Neurosci. 1993;16:73–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

