Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter. A specific role for sialic acid residues
- PMID: 12944413
- PMCID: PMC3042025
- DOI: 10.1074/jbc.M306360200
Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter. A specific role for sialic acid residues
Retraction in
-
Withdrawal: Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter: a specific role for sialic acid residues.J Biol Chem. 2019 Jun 14;294(24):9657. doi: 10.1074/jbc.W119.009466. J Biol Chem. 2019. PMID: 31201245 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern: Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter: A specific role for sialic acid residues.J Biol Chem. 2019 Mar 29;294(13):5210. doi: 10.1074/jbc.EC119.008297. J Biol Chem. 2019. PMID: 30926758 Free PMC article. No abstract available.
Abstract
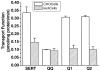
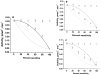
The serotonin transporter (SERT) is an oligomeric glycoprotein with two sialic acid residues on each of two complex oligosaccharide molecules. In this study, we investigated the contribution of N-glycosyl modification to the structure and function of SERT in two model systems: wild-type SERT expressed in sialic acid-defective Lec4 Chinese hamster ovary (CHO) cells and a mutant form (after site-directed mutagenesis of Asn-208 and Asn-217 to Gln) of SERT, QQ, expressed in parental CHO cells. In both systems, SERT monomers required modification with both complex oligosaccharide residues to associate with each other and to function in homo-oligomeric forms. However, defects in sialylated N-glycans did not alter surface expression of the SERT protein. Furthermore, in heterologous (CHO and Lec4 cells) and endogenous (placental choriocarcinoma JAR cells) expression systems, we tested whether glycosyl modification also manipulates the hetero-oligomeric interactions of SERT, specifically with myosin IIA. SERT is phosphorylated by cGMP-dependent protein kinase G through interactions with anchoring proteins, and myosin is a protein kinase G-anchoring protein. A physical interaction between myosin and SERT was apparent; however, defects in sialylated N-glycans impaired association of SERT with myosin as well as the stimulation of the serotonin uptake function in the cGMP-dependent pathway. We propose that sialylated N-glycans provide a favorable conformation to SERT that allows the transporter to function most efficiently via its protein-protein interactions.
Figures
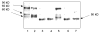




References
-
- Blakely R, Berson H, Fremeau R, Jr, Caron M, Peek M, Priace H, Bradley C. Nature. 1991;354:66–70. - PubMed
-
- Hoffman B, Mezey E, Browstein M. Science. 1991;254:579–580. - PubMed
-
- Uhl G. Trends Neurosci. 1992;15:265–268. - PubMed
-
- Kanner B. J Exp Biol. 1994;196:237–249. - PubMed
-
- Amara S, Kuhar M. Annu Rev Neurosci. 1993;16:73–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

