Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination
- PMID: 12944468
- PMCID: PMC193719
- DOI: 10.1128/MCB.23.18.6396-6405.2003
Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination
Abstract
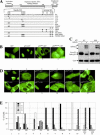
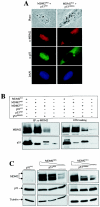
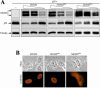
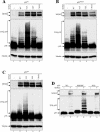
As a shuttling protein, p53 is constantly transported through the nuclear pore complex. p53 nucleocytoplasmic transport is carried out by a bipartite nuclear localization signal (NLS) located at its C-terminal domain and two nuclear export signals (NES) located in its N- and C-terminal regions, respectively. The role of nucleocytoplasmic shuttling in p53 ubiquitination and degradation has been a subject of debate. Here we show that the two basic amino acid groups in the p53 bipartite NLS function collaboratively to import p53. Mutations disrupting individual amino acids in the NLS, although causing accumulation of p53 in the cytoplasm to various degrees, reduce but do not eliminate the NLS activity, and these mutants remain sensitive to MDM2 degradation. However, disrupting both parts of the bipartite NLS completely blocks p53 from entering the nucleus and causes p53 to become resistant to MDM2-mediated degradation. Similarly, mutations disrupting four conserved hydrophobic amino acids in the p53 C-terminal NES block p53 export and prohibit it from MDM2 degradation. We also show that colocalization of a nonshuttling p53 with MDM2 either in the nucleus or in the cytoplasm is sufficient for MDM2-induced p53 polyubiquitination but not degradation. Our data provide new insight into the mechanism and regulation of p53 nucleocytoplasmic shuttling and degradation.
Figures



Similar articles
-
[Nuclear import of p53 in relation to MDM2-mediated degradation and ubiquitination].Zhonghua Zhong Liu Za Zhi. 2005 Feb;27(2):86-9. Zhonghua Zhong Liu Za Zhi. 2005. PMID: 15946545 Chinese.
-
Mono- versus polyubiquitination: differential control of p53 fate by Mdm2.Science. 2003 Dec 12;302(5652):1972-5. doi: 10.1126/science.1091362. Science. 2003. PMID: 14671306
-
Regulation of p53 nuclear export through sequential changes in conformation and ubiquitination.J Biol Chem. 2007 May 11;282(19):14616-25. doi: 10.1074/jbc.M610515200. Epub 2007 Mar 19. J Biol Chem. 2007. PMID: 17371868
-
Regulation of p53 localization.Eur J Biochem. 2001 May;268(10):2779-83. doi: 10.1046/j.1432-1327.2001.02227.x. Eur J Biochem. 2001. PMID: 11358492 Review.
-
Control of p53 ubiquitination and nuclear export by MDM2 and ARF.Cell Growth Differ. 2001 Apr;12(4):175-86. Cell Growth Differ. 2001. PMID: 11331246 Review.
Cited by
-
Identification and functional analysis of NOL7 nuclear and nucleolar localization signals.BMC Cell Biol. 2010 Sep 27;11:74. doi: 10.1186/1471-2121-11-74. BMC Cell Biol. 2010. PMID: 20875127 Free PMC article.
-
Phosphoproteomics of primary AML patient samples reveals rationale for AKT combination therapy and p53 context to overcome selinexor resistance.Cell Rep. 2022 Aug 9;40(6):111177. doi: 10.1016/j.celrep.2022.111177. Cell Rep. 2022. PMID: 35947955 Free PMC article.
-
PSPC1 is a new contextual determinant of aberrant subcellular translocation of oncogenes in tumor progression.J Biomed Sci. 2021 Aug 2;28(1):57. doi: 10.1186/s12929-021-00753-3. J Biomed Sci. 2021. PMID: 34340703 Free PMC article. Review.
-
Correlation of p53 oligomeric status and its subcellular localization in the presence of the AML-associated NPM mutant.PLoS One. 2025 May 7;20(5):e0322096. doi: 10.1371/journal.pone.0322096. eCollection 2025. PLoS One. 2025. PMID: 40334261 Free PMC article.
-
Proteins of the S100 family regulate the oligomerization of p53 tumor suppressor.Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4735-40. doi: 10.1073/pnas.0501459102. Epub 2005 Mar 21. Proc Natl Acad Sci U S A. 2005. PMID: 15781852 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
