Highly restricted localization of RNA polymerase II within a locus control region of a tissue-specific chromatin domain
- PMID: 12944475
- PMCID: PMC193707
- DOI: 10.1128/MCB.23.18.6484-6493.2003
Highly restricted localization of RNA polymerase II within a locus control region of a tissue-specific chromatin domain
Abstract
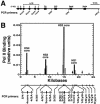
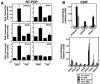
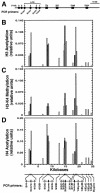
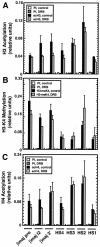
RNA polymerase II (Pol II) can associate with regulatory elements far from promoters. For the murine beta-globin locus, Pol II binds the beta-globin locus control region (LCR) far upstream of the beta-globin promoters, independent of recruitment to and activation of the betamajor promoter. We describe here an analysis of where Pol II resides within the LCR, how it is recruited to the LCR, and the functional consequences of recruitment. High-resolution analysis of the distribution of Pol II revealed that Pol II binding within the LCR is restricted to the hypersensitive sites. Blocking elongation eliminated the synthesis of genic and extragenic transcripts and eliminated Pol II from the betamajor open reading frame. However, the elongation blockade did not redistribute Pol II at the hypersensitive sites, suggesting that Pol II is recruited to these sites. The distribution of Pol II did not strictly correlate with the distributions of histone acetylation and methylation. As Pol II associates with histone-modifying enzymes, Pol II tracking might be critical for establishing and maintaining broad histone modification patterns. However, blocking elongation did not disrupt the histone modification pattern of the beta-globin locus, indicating that Pol II tracking is not required to maintain the pattern.
Figures



References
-
- Agalioti, T., G. Chen, and D. Thanos. 2002. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111:381-392. - PubMed
-
- Bender, M. A., M. Bulger, J. Close, and M. Groudine. 2000. Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol. Cell 5:387-393. - PubMed
-
- Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
