Molecular mechanisms associated with the regulation of apoptosis by the two alternatively spliced products of c-Myb
- PMID: 12944488
- PMCID: PMC193713
- DOI: 10.1128/MCB.23.18.6631-6645.2003
Molecular mechanisms associated with the regulation of apoptosis by the two alternatively spliced products of c-Myb
Abstract
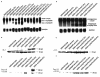
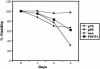
The c-myb proto-oncogene encodes two alternatively spliced mRNAs, which in turn code for proteins of 75 kDa and 89 kDa. It is at present unclear whether the two isoforms of c-Myb perform identical functions or whether they mediate different biological effects. To assess their role in apoptotic death of hematopoietic cells, we expressed the two isoforms of c-Myb in the murine myeloid cell lines 32Dcl3 and FDCP1. Our results show that while ectopic overexpression of p75 c-Myb results in the acceleration of cell death, similar overexpression of p89 c-Myb results in the protection of cells from apoptotic death. An analysis of gene expression changes with mouse cDNA expression arrays revealed that while p75 c-Myb blocked the expression of glutathione S-transferase micro mRNA, p89 c-Myb greatly enhanced the expression of this gene. These results were further confirmed by Northern blot analysis. Ectopic overexpression of the glutathione S-transferase micro gene in 32Dcl3 cells resulted in protection of cells from interleukin-3 withdrawal-induced cell death similar to that seen with the ectopic overexpression of p89 c-Myb. These results suggest that the two isoforms of c-Myb differentially regulate apoptotic death of myeloid cells through differential regulation of glutathione S-transferase micro gene expression.
Figures







References
-
- Askew, D. S., R. A. Ashmun, B. C. Simmons, and J. L. Cleveland. 1991. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6:1915-1922. - PubMed
-
- Baker, S. J., and E. P. Reddy. 1998. Modulation of life and death by the TNF receptor superfamily. Oncogene 17:3261-3270. - PubMed
-
- Bergmann, A., J. Agapite, and H. Steller. 1998. Mechanisms and control of programmed cell death in invertebrates. Oncogene 17:3215-3223. - PubMed
-
- Biedenkapp, H., U. Borgmeyer, A. E. Sippel, and K. H. Klempnauer. 1988. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature 335:835-837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
