Involvement of nucleocytoplasmic shuttling of yeast Nap1 in mitotic progression
- PMID: 12944491
- PMCID: PMC193709
- DOI: 10.1128/MCB.23.18.6672-6684.2003
Involvement of nucleocytoplasmic shuttling of yeast Nap1 in mitotic progression
Abstract
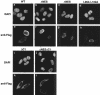
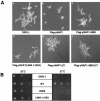
Nucleosome assembly protein 1 (Nap1) is widely conserved from yeasts to humans and facilitates nucleosome formation in vitro as a histone chaperone. Nap1 is generally localized in the cytoplasm, except that subcellular localization of Drosophila melanogaster Nap1 is dynamically regulated between the cytoplasm and nucleus during early development. The cytoplasmic localization of Nap1 is seemingly incompatible with the proposed role of Nap1 in nucleosome formation, which should occur in the nucleus. Here, we have examined the roles of a putative nuclear export signal (NES) sequence in yeast Nap1 (yNap1). yNap1 mutants lacking the NES-like sequence were localized predominantly in the nucleus. Deletion of NAP1 in cells harboring a single mitotic cyclin gene is known to cause mitotic delay and temperature-sensitive growth. A wild-type NAP1 complemented these phenotypes while nap1 mutant genes lacking the NES-like sequence or carboxy-terminal region did not. These and other results suggest that yNap1 is a nucleocytoplasmic shuttling protein and that its shuttling is important for yNap1 function during mitotic progression. This study also provides a possible explanation for Nap1's involvement in nucleosome assembly and/or remodeling in the nucleus.
Figures

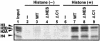




References
-
- Aalfs, J. D., and Kingston, R. E. 2000. What does ‘chromatin remodeling’ mean? Trends Biochem. Sci. 25:548-555. - PubMed
-
- Adams, C. R., and R. T. Kamakaka. 1999. Chromatin assembly: biochemical identities and genetic redundancy. Curr. Opin. Genet. Dev. 9:185-190. - PubMed
-
- Bortvin, A., and F. Winston. 1996. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science 272:1473-1476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
