Targeted disruption of the inosine 5'-monophosphate dehydrogenase type I gene in mice
- PMID: 12944494
- PMCID: PMC193693
- DOI: 10.1128/MCB.23.18.6702-6712.2003
Targeted disruption of the inosine 5'-monophosphate dehydrogenase type I gene in mice
Abstract
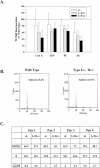
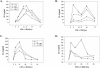
Inosine 5'-monophosphate dehydrogenase (IMPDH) is the critical, rate-limiting enzyme in the de novo biosynthesis pathway for guanine nucleotides. Two separate isoenzymes, designated IMPDH types I and II, contribute to IMPDH activity. An additional pathway salvages guanine through the activity of hypoxanthine-guanine phosphoribosyltransferase (HPRT) to supply the cell with guanine nucleotides. In order to better understand the relative contributions of IMPDH types I and II and HPRT to normal biological function, a mouse deficient in IMPDH type I was generated by standard gene-targeting techniques and bred to mice deficient in HPRT or heterozygous for IMPDH type II. T-cell activation in response to anti-CD3 plus anti-CD28 antibodies was significantly impaired in both single- and double-knockout mice, whereas a more general inhibition of proliferation in response to other T- and B-cell mitogens was observed only in mice deficient in both enzymes. In addition, IMPDH type I(-/-) HPRT(-/0) splenocytes showed reduced interleukin-4 production and impaired cytolytic activity after antibody activation, indicating an important role for guanine salvage in supplementing the de novo synthesis of guanine nucleotides. We conclude that both IMPDH and HPRT activities contribute to normal T-lymphocyte activation and function.
Figures


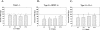
Similar articles
-
Inhibition of T lymphocyte activation in mice heterozygous for loss of the IMPDH II gene.J Clin Invest. 2000 Aug;106(4):599-606. doi: 10.1172/JCI8669. J Clin Invest. 2000. PMID: 10953035 Free PMC article.
-
Inosine-5'-monophosphate dehydrogenase: regulation of expression and role in cellular proliferation and T lymphocyte activation.Prog Nucleic Acid Res Mol Biol. 1998;61:181-209. doi: 10.1016/s0079-6603(08)60827-2. Prog Nucleic Acid Res Mol Biol. 1998. PMID: 9752721 Review.
-
Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression.J Immunol. 1994 Feb 1;152(3):984-91. J Immunol. 1994. PMID: 7905505
-
Regulation of inosine monophosphate dehydrogenase type I and type II isoforms in human lymphocytes.Biochem Pharmacol. 2004 Feb 15;67(4):767-76. doi: 10.1016/j.bcp.2003.09.043. Biochem Pharmacol. 2004. PMID: 14757177
-
Consequences of IMP dehydrogenase inhibition, and its relationship to cancer and apoptosis.Curr Med Chem. 1999 Jul;6(7):561-74. Curr Med Chem. 1999. PMID: 10390601 Review.
Cited by
-
Cryo-EM structures demonstrate human IMPDH2 filament assembly tunes allosteric regulation.Elife. 2020 Jan 30;9:e53243. doi: 10.7554/eLife.53243. Elife. 2020. PMID: 31999252 Free PMC article.
-
IMP/GTP balance modulates cytoophidium assembly and IMPDH activity.Cell Div. 2018 Jun 15;13:5. doi: 10.1186/s13008-018-0038-0. eCollection 2018. Cell Div. 2018. PMID: 29946345 Free PMC article.
-
GTP metabolic reprogramming by IMPDH2: unlocking cancer cells' fuelling mechanism.J Biochem. 2020 Oct 1;168(4):319-328. doi: 10.1093/jb/mvaa085. J Biochem. 2020. PMID: 32702086 Free PMC article. Review.
-
Coordinated Formation of IMPDH2 Cytoophidium in Mouse Oocytes and Granulosa Cells.Front Cell Dev Biol. 2021 May 28;9:690536. doi: 10.3389/fcell.2021.690536. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34124077 Free PMC article.
-
The raspberry Gene Is Involved in the Regulation of the Cellular Immune Response in Drosophila melanogaster.PLoS One. 2016 Mar 4;11(3):e0150910. doi: 10.1371/journal.pone.0150910. eCollection 2016. PLoS One. 2016. PMID: 26942456 Free PMC article.
References
-
- Carr, S. F., E. Papp, J. C. Wu, and Y. Natsumeda. 1993. Characterization of human type I and type II IMP dehydrogenases. J. Biol. Chem. 268:27286-27290. - PubMed
-
- Carrera, A. C., L. Rodriguez-Borlado, C. Martinez-Alonso, and I. Merida. 1994. T cell receptor-associated α-phosphatidylinositol 3-kinase becomes activated by T cell receptor cross-linking and requires pp56lck. J. Biol. Chem. 269:19435-19440. - PubMed
-
- Collart, F. R., C. B. Chubb, B. L. Mirkin, and E. Huberman. 1992. Increased inosine-5′-phosphate dehydrogenase gene expression in solid tumor tissues and tumor cell lines. Cancer Res. 52:5826-5828. - PubMed
-
- Collart, F. R., and E. Huberman. 1988. Cloning and sequence analysis of the human and Chinese hamster inosine-5′-monophosphate dehydrogenase cDNAs. J. Biol. Chem. 263:15769-15772. - PubMed
-
- Dayton, J. S., T. Lindsten, C. B. Thompson, and B. S. Mitchell. 1994. Effects of human T lymphocyte activation on inosine monophosphate dehydrogenase expression. J. Immunol. 152:984-991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
