Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons
- PMID: 12944503
- PMCID: PMC6740608
- DOI: 10.1523/JNEUROSCI.23-21-07750.2003
Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons
Abstract
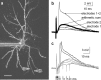
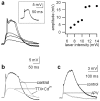
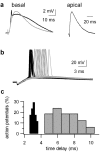
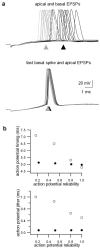
The ability of cortical neurons to perform temporally accurate computations has been shown to be important for encoding of information in the cortex; however, cortical neurons are expected to be imprecise temporal encoders because of the stochastic nature of synaptic transmission and ion channel gating, dendritic filtering, and background synaptic noise. Here we show for the first time that fast local spikes in basal dendrites can serve to improve the temporal precision of neuronal output. Integration of coactivated, spatially distributed synaptic inputs produces temporally imprecise output action potentials within a time window of several milliseconds. In contrast, integration of closely spaced basal inputs initiates local dendritic spikes that amplify and sharpen the summed somatic potential. In turn, these fast basal spikes allow precise timing of output action potentials with submillisecond temporal jitter over a wide range of activation intensities and background synaptic noise. Our findings indicate that fast spikes initiated in individual basal dendrites can serve as precise "timers" of output action potentials in various network activity states and thus may contribute to temporal coding in the cortex.
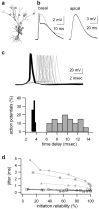
Figures

References
-
- Abeles M ( 1990) Corticonics. Cambridge, UK: Cambridge UP.
-
- Archie KA, Mel BW ( 2000) A model for intradendritic computation of binocular disparity. Nat Neurosci 3: 54-63. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A ( 1996) Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868-1871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
