Increased morphological diversity of microglia in the activated hypothalamic supraoptic nucleus
- PMID: 12944504
- PMCID: PMC6740605
- DOI: 10.1523/JNEUROSCI.23-21-07759.2003
Increased morphological diversity of microglia in the activated hypothalamic supraoptic nucleus
Abstract
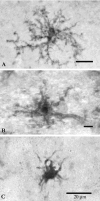
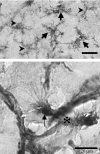
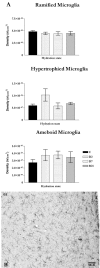
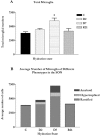
Microglia are the immune cells of the CNS. In the normal adult mammalian brain, the majority of these cells is quiescent and exhibits a ramified morphology. Microglia are perhaps best known for their swift transformation to an activated ameboid morphology in response to pathological insults. Here we have observed the responsiveness of these cells to events surrounding the normal activation of neurosecretory neurons in the hypothalamic supraoptic nucleus (SON), a well studied model of structural plasticity in the CNS. Neurons in the SON were activated by substituting 2% saline for drinking water. Brain sections were collected from four experimental groups [controls (C), 2 d-dehydrated (2D), 7 d-dehydrated (D7), and 7 d-dehydrated/21 d-rehydrated animals (R21)] and stained with Isolectin-B4-HRP to visualize microglial cells. Based on morphological criteria, we quantified ramified, hypertrophied, and ameboid microglia using unbiased stereological techniques. Statistical analyses showed significant increases in the number of hypertrophied microglia in the D2 and D7 groups. Moreover, there was a significant increase in the number of ameboid microglia in the D7 group. No changes were seen across conditions in the number of ramified cells, nor did we observe any significant phenotypic changes in a control area of the cingulate gyrus. Hence, increased morphological diversity of microglia was found specifically in the SON and was reversible with the cessation of stimulation. These results indicate that phenotypic plasticity of microglia may be a feature of the normal structural remodeling that accompanies neuronal activation in addition to the activation that accompanies brain pathology.
Figures



References
-
- Andrew RD, MacVicar BA, Dudek FE, Hatton GI ( 1981) Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science 211: 1187-1189. - PubMed
-
- Ayoub A, Salm AK ( 2000) Morphological plasticity of microglia in the activated supraoptic nucleus. Soc Neurosci Abstr 26: 1943. 2000.
-
- Banati RB, Myers R, Kreutzberg GW ( 1997) PK (“peripheral benzodiazepine”)-binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol 26: 77-82. - PubMed
-
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR ( 1964) Chemical and anatomical elasticity of brain. Science 146: 610-619. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
