Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility
- PMID: 12944505
- PMCID: PMC6740602
- DOI: 10.1523/JNEUROSCI.23-21-07767.2003
Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility
Abstract
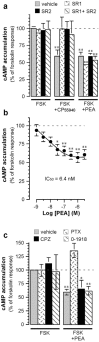
Focal cerebral ischemia (FCI) induces rapid neuronal death in the ischemic core, which gradually expands toward the penumbra, partly as the result of a neuroinflammatory response. It is known that propagation of neuroinflammation involves microglial cells, the resident macrophages of the brain, which are highly motile when activated by specific signals. However, the signals that increase microglial cell motility in response to FCI remain mostly elusive. Here, we tested the hypothesis that endocannabinoids mediate neuroinflammation propagation by increasing microglial cell motility. We found that, in mouse cerebral cortex, FCI greatly increases palmitoylethanolamide (PEA), only moderately increases anandamide [arachidonylethanolamide (AEA)], and does not affect 2-arachidonoylglycerol levels. We also found that PEA potentiates AEA-induced microglial cell migration, without affecting other steps of microglial activation, such as proliferation, particle engulfment, and nitric oxide production. This potentiation of microglial cell migration by PEA involves reduction in cAMP levels. In line with this, we provide evidence that PEA acts through Gi/o-coupled receptors. Interestingly, these receptors engaged by PEA are pharmacologically distinct from CB1 and CB2 cannabinoid receptors, as well as from the WIN and abn-CBD (abnormal-cannabidiol) receptors, two recently identified cannabinoid receptors. Our results show that PEA and AEA increase after FCI and synergistically enhance microglial cell motility. Because such a response could participate in the propagation of the FCI-induced neuroinflammation within the CNS, and because PEA is likely to act through its own receptor, a better understanding of the receptor engaged by PEA may help guide the search for improved therapies against neuroinflammation.
Figures


Similar articles
-
Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells.Biochem J. 2001 Aug 15;358(Pt 1):249-55. doi: 10.1042/0264-6021:3580249. Biochem J. 2001. PMID: 11485574 Free PMC article.
-
Nonpsychotropic cannabinoid receptors regulate microglial cell migration.J Neurosci. 2003 Feb 15;23(4):1398-405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. J Neurosci. 2003. PMID: 12598628 Free PMC article.
-
Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB(1) and CB(2)-like receptors.Pain. 2002 May;97(1-2):11-21. doi: 10.1016/s0304-3959(01)00419-5. Pain. 2002. PMID: 12031775
-
Cannabimimetic fatty acid derivatives in cancer and inflammation.Prostaglandins Other Lipid Mediat. 2000 Apr;61(1-2):43-61. doi: 10.1016/s0090-6980(00)00054-x. Prostaglandins Other Lipid Mediat. 2000. PMID: 10785541 Review.
-
Cellular transport of anandamide, 2-arachidonoylglycerol and palmitoylethanolamide--targets for drug development?Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):193-200. doi: 10.1054/plef.2001.0357. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052035 Review.
Cited by
-
Endocannabinoid signalling in innate and adaptive immunity.Immunology. 2015 Mar;144(3):352-364. doi: 10.1111/imm.12441. Immunology. 2015. PMID: 25585882 Free PMC article. Review.
-
Cannabinoid CB1 receptor stimulation affords neuroprotection in MPTP-induced neurotoxicity by attenuating S100B up-regulation in vitro.J Mol Med (Berl). 2007 Dec;85(12):1379-92. doi: 10.1007/s00109-007-0233-y. Epub 2007 Jul 17. J Mol Med (Berl). 2007. PMID: 17639288
-
Endocannabinoids and reactive nitrogen and oxygen species in neuropathologies.J Neuroimmune Pharmacol. 2006 Sep;1(3):305-16. doi: 10.1007/s11481-006-9022-6. Epub 2006 Jun 24. J Neuroimmune Pharmacol. 2006. PMID: 18040807 Review.
-
Role of integrating cannabinoids and the endocannabinoid system in neonatal hypoxic-ischaemic encephalopathy.Front Mol Neurosci. 2023 Apr 12;16:1152167. doi: 10.3389/fnmol.2023.1152167. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37122621 Free PMC article. Review.
-
A randomized controlled trial assessing the safety and efficacy of palmitoylethanolamide for treating diabetic-related peripheral neuropathic pain.Inflammopharmacology. 2022 Dec;30(6):2063-2077. doi: 10.1007/s10787-022-01033-8. Epub 2022 Sep 4. Inflammopharmacology. 2022. PMID: 36057884 Free PMC article. Clinical Trial.
References
-
- Baker D, Pryce G, Croxford LJ, Brown P, Pertwee RG, Makriyannis A, Knanolkar A, Layward L, Fezza F, Bisogno T, DiMarzo V ( 2001) Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J 15: 300-302. - PubMed
-
- Barnum SR, Ames RS, Maycox PR, Hadingham SJ, Meakin J, Harrison D, Parsons AA ( 2002) Expression of the complement C3a and C5a receptors after permanent focal ischemia: an alternative interpretation. Glia 38: 169-173. - PubMed
-
- Bayewitch M, Avidor-Reiss T, Levy R, Barg J, Mechoulam R, Vogel Z ( 1995) The peripheral cannabinoid receptor: adenylate cyclase inhibition and G protein coupling. FEBS Lett 375: 143-147. - PubMed
-
- Becher B, Prat A, Antel JP ( 2000) Brain-immune connection: immunoregulatory properties of CNS-resident cells. Glia 29: 293-304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
