Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats
- PMID: 12944508
- PMCID: PMC6740614
- DOI: 10.1523/JNEUROSCI.23-21-07789.2003
Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats
Abstract
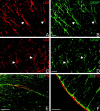
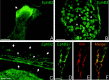
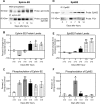
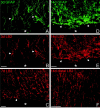
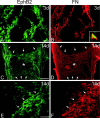
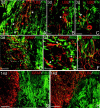
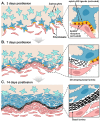
The present study provides the first evidence that signaling occurs between B-ephrins and EphB receptors in the adult CNS in response to injury. Specifically, our combined histological and biochemical data indicate that two members of the B-class of ephrins and Eph receptors, ephrin-B2 and EphB2, are expressed by astrocytes and meningeal fibroblasts, respectively, in the adult spinal cord. In response to thoracic spinal cord transection lesions, ephrin-B2 and EphB2 protein levels exhibit an initial decrease (1 d after lesion), followed by a significant increase by day 14. Immunohistochemical data indicate that ephrin-B2 is expressed by reactive CNS astrocytes, and EphB2 is present on fibroblasts invading the lesion site from the adjacent meninges. During the first 3 d after injury, there is intermingling of ephrin-B2-expressing reactive astrocytes at the lesion surface with EphB2-containing fibroblasts that is concurrent with bidirectional activation (phosphorylation) of ephrin-B2 and EphB2. By 7 d, both cell types are establishing restricted cellular domains containing dense networks of cells and interweaving processes. This astroglial-meningeal fibroblast scar is fully developed by day 14 when there is strict segregation of ephrin-B2-expressing astrocytes from EphB2-positive meningeal fibroblasts. These morphological changes are concomitant with a simultaneous decrease in ephrin-B2 and EphB2 activation. These observations provide strong evidence that cell contact-mediated bidirectional signaling between ephrin-B2 on reactive astrocytes and EphB2 on meningeal fibroblasts is an early event in the cellular cascades that result in the development of the glial scar and the exclusion of meningeal fibroblasts from the injured spinal cord.
Figures
References
-
- Abnet K, Fawcett JW, Dunnett SB ( 1991) Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res Dev Brain Res 59: 187-196. - PubMed
-
- Berry M, Maxwell WL, Logan A, Mathewson A, McConnell P, Ashhurst DE, Thomas GH ( 1983) Deposition of scar tissue in the central nervous system. Acta Neurochir (Wien) [Suppl] 32: 31-53. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB ( 2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636-640. - PubMed
-
- Bruckner K, Pasquale EB, Klein R ( 1997) Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275: 1640-1643. - PubMed
-
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R ( 1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22: 511-524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
