Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral-induced brain inflammation, but delays immune system-mediated elimination of transgene expression
- PMID: 12946313
- PMCID: PMC2913593
- DOI: 10.1016/s1525-0016(03)00178-3
Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral-induced brain inflammation, but delays immune system-mediated elimination of transgene expression
Abstract
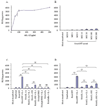
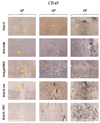
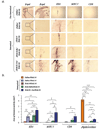
Despite their ability to provide long-term transgene expression in the central nervous system of naïve hosts, the use of first-generation adenovirus (Ad) vectors for the treatment of chronic neurological disorders is limited by peripheral immunization, which stimulates anti-adenovirus immune responses and causes severe inflammation in the central nervous system (CNS) and elimination of transgene expression. The purpose of this study was to investigate the roles of NF-kappaB and interleukin-1 (IL-1) during inflammatory responses to Ads in the CNS of naïve and preimmunized rats. We assessed activation of macrophages/microglia, up-regulation of MHC I expression, infiltration of leukocytes, and transgene expression following delivery of Ads to the rat striatum. After delivery of increasing doses of adenoviral vectors expressing various anti-inflammatory agents (e.g., NF-kappaB or IL-1 inhibitors) to naïve rats, no reduction in Ad-mediated CNS inflammation was seen 1 week after delivery of Ads, compared to a control Ad.hCMV.beta-galactosidase (RAd.35) virus. We then assessed CNS inflammation and transgene expression at a time when control transgene expression would be completely eliminated, i.e., 1 month post-vector injection into the brain. This would optimize the assessment of an anti-inflammatory agent expressed by an adenoviral vector that could either delay or diminish immune system-mediated elimination of transgene expression. As expected, at 1 month postinfection, control preimmunized rats receiving Ad.mCMV.beta-galactosidase (RAd.36)/saline or RAd.36/Ad.null (RAd.0) showed complete elimination of beta-galactosidase expression in the brain and levels of inflammation comparable to those of naïve animals. However, animals injected with RAd.36 in combination with Ads expressing NF-kappaB or IL-1 inhibitors showed a delayed elimination of beta-galactosidase compared to controls. As predicted, the extended presence of transgene expression was accompanied by increased levels of CNS inflammation. This suggests that blocking NF-kappaB or IL-1 delays, albeit partially, transgene elimination in the presence of a preexisting systemic immune response. Prolonged transgene expression is predicted to extend concurrent brain inflammation, as noted earlier. Taken together these data demonstrate a role for NF-kappaB and IL-1 in immune system-mediated elimination of Ad-mediated CNS transgene expression.
Figures



Similar articles
-
Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice.Gene Ther. 2007 Mar;14(6):533-44. doi: 10.1038/sj.gt.3302885. Epub 2006 Nov 16. Gene Ther. 2007. PMID: 17109009
-
Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses.Hum Gene Ther. 2005 Jun;16(6):741-51. doi: 10.1089/hum.2005.16.741. Hum Gene Ther. 2005. PMID: 15960605 Free PMC article.
-
Inhibition of NF-kappaB activation in combination with bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver.J Virol. 1998 Nov;72(11):9267-77. doi: 10.1128/JVI.72.11.9267-9277.1998. J Virol. 1998. PMID: 9765474 Free PMC article.
-
Alveolar macrophages and T cells from sarcoid, but not normal lung, are permissive to adenovirus infection and allow analysis of NF-kappa b-dependent signaling pathways.Am J Respir Cell Mol Biol. 2001 Aug;25(2):141-9. doi: 10.1165/ajrcmb.25.2.4327. Am J Respir Cell Mol Biol. 2001. PMID: 11509322
-
Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain.J Neurosci. 1999 Feb 15;19(4):1517-23. doi: 10.1523/JNEUROSCI.19-04-01517.1999. J Neurosci. 1999. PMID: 9952427 Free PMC article.
Cited by
-
Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain.Am J Pathol. 2004 Nov;165(5):1827-37. doi: 10.1016/S0002-9440(10)63438-4. Am J Pathol. 2004. PMID: 15509551 Free PMC article.
-
The thymus-neuroendocrine axis: physiology, molecular biology, and therapeutic potential of the thymic peptide thymulin.Ann N Y Acad Sci. 2009 Feb;1153:98-106. doi: 10.1111/j.1749-6632.2008.03964.x. Ann N Y Acad Sci. 2009. PMID: 19236333 Free PMC article. Review.
-
Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model.Cancer Gene Ther. 2004 Nov;11(11):721-32. doi: 10.1038/sj.cgt.7700783. Cancer Gene Ther. 2004. PMID: 15475879 Free PMC article.
References
-
- Akli S, et al. Transfer of a foreign gene into the brain using adenovirus vectors. Nat. Genet. 1993;3:224–228. - PubMed
-
- Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 1993;3:219–223. - PubMed
-
- Le Gal La Salle G, et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. - PubMed
-
- Geddes BJ, Harding TC, Lightman SL, Uney JB. Long-term gene therapy in the CNS: Reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nat. Med. 1997;3:1402–1404. - PubMed
-
- Navarro V, et al. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Ther. 1999;6:1884–1892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

