How protein thermodynamics and folding mechanisms are altered by the chaperonin cage: molecular simulations
- PMID: 12947041
- PMCID: PMC208763
- DOI: 10.1073/pnas.1831920100
How protein thermodynamics and folding mechanisms are altered by the chaperonin cage: molecular simulations
Abstract
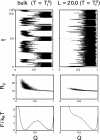
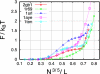
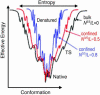
How the Escherichia coli GroEL/ES chaperonin assists folding of a substrate protein remains to be uncovered. Recently, it was suggested that confinement into the chaperonin cage itself can significantly accelerate folding of a substrate. Performing comprehensive molecular simulations of eight proteins confined into various sizes L of chaperonin-like cage, we explore how and to what extent protein thermodynamics and folding mechanisms are altered by the cage. We show that a substrate protein is remarkably stabilized by confinement; the estimated increase in denaturation temperature DeltaTf is as large as approximately 60 degrees C. For a protein of size R0, the stabilization DeltaTf scales as (R0/L)nu, where nu approximately 3, which is consistent with a mean field theory of polymer. We also found significant free energy cost of confining a protein, which increases with R0/L, indicating that the confinement requires external work provided by the chaperonin system. In kinetic study, we show the folding is accelerated in a modestly well confined case, which is consistent with a recent experimental result on ribulose-1,5-bisphosphate carboxylase-oxygenase folding and simulation results of a beta hairpin. Interestingly, the acceleration of folding is likely to be larger for a protein with more complex topology, as quantified by the contact order. We also show how ensemble of folding pathways are altered by the chaperonin-like cage calculating a variant of value used in the study of spontaneous folding.
Figures



Comment in
-
Caging helps proteins fold.Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11195-7. doi: 10.1073/pnas.2035072100. Epub 2003 Sep 23. Proc Natl Acad Sci U S A. 2003. PMID: 14506295 Free PMC article. No abstract available.
References
-
- Sigler, P. B., Xu, Z., Rye, H. S., Burston, S. G., Fenton, W. A. & Horwich, A. L. (1998) Annu. Rev. Biochem. 67, 581–608. - PubMed
-
- Hartl, F. U. & Hayer-Hartl, M. (2002) Science 295, 1852–1858. - PubMed
-
- Braig, K., Otwinowski, Z., Hegde, R., Boisvert, D. C., Joachimiak, A., Horwich, A. L. & Sigler, P. B. (1994) Nature 371, 578–586. - PubMed
-
- Xu, Z., Horwich, A. L. & Sigler, P. B. (1997) Nature 388, 741–750. - PubMed
-
- Weissman, J. S., Kashi, Y., Fenton, W. A. & Horwich, A. L. (1994) Cell 78, 693–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

