Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor
- PMID: 12949227
- PMCID: PMC2343443
- DOI: 10.1113/jphysiol.2003.048405
Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor
Abstract
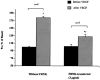
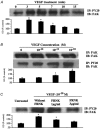
Focal adhesion kinase (FAK) is known to mediate endothelial cell adhesion and migration in response to vascular endothelial growth factor (VEGF). The aim of this study was to explore a potential role for FAK in VEGF regulation of microvascular endothelial barrier function. The apparent permeability coefficient of albumin (Pa) was measured in intact isolated porcine coronary venules. Treating the vessels with VEGF induced a time- and concentration-dependent increase in Pa. Inhibition of FAK through direct delivery of FAK-related non-kinase (FRNK) into venular endothelium did not alter basal barrier function but significantly attenuated VEGF-elicited hyperpermeability. Furthermore, cultured human umbilical vein endothelial monolayers displayed a similar hyperpermeability response to VEGF which was greatly attenuated by FRNK. Western blot analysis showed that VEGF promoted FAK phosphorylation in a time course correlating with that of venular hyperpermeability. The phosphorylation response was blocked by FRNK treatment. In addition, VEGF stimulation caused a significant morphological change of FAK from a punctate pattern to an elongated, dash-like staining that aligned with the longitudinal axis of the cells. Taken together, the results suggest that FAK contributes to VEGF-elicited vascular hyperpermeability. Phosphorylation of FAK may play an important role in the signal transduction of vascular barrier response to VEGF.
Figures


Similar articles
-
Focal adhesion kinase in neutrophil-induced microvascular hyperpermeability.Microcirculation. 2005 Mar;12(2):223-32. doi: 10.1080/10739680590905251. Microcirculation. 2005. PMID: 15824042
-
Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability.Am J Physiol. 1999 Feb;276(2):H535-42. doi: 10.1152/ajpheart.1999.276.2.H535. Am J Physiol. 1999. PMID: 9950855
-
The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules.J Physiol. 2005 Feb 15;563(Pt 1):95-104. doi: 10.1113/jphysiol.2004.076075. Epub 2004 Nov 11. J Physiol. 2005. PMID: 15539400 Free PMC article.
-
Regulation of endothelial cell function BY FAK and PYK2.Front Biosci. 2004 May 1;9:1254-66. doi: 10.2741/1239. Front Biosci. 2004. PMID: 14977542 Review.
-
Protein kinase signaling in the modulation of microvascular permeability.Vascul Pharmacol. 2002 Nov;39(4-5):213-23. doi: 10.1016/s1537-1891(03)00010-7. Vascul Pharmacol. 2002. PMID: 12747961 Review.
Cited by
-
Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function.Microvasc Res. 2012 Jan;83(1):31-44. doi: 10.1016/j.mvr.2011.05.004. Epub 2011 May 20. Microvasc Res. 2012. PMID: 21640127 Free PMC article. Review.
-
Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions.Microvasc Res. 2012 Jan;83(1):71-81. doi: 10.1016/j.mvr.2011.06.007. Epub 2011 Jun 29. Microvasc Res. 2012. PMID: 21741394 Free PMC article. Review.
-
Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases.Am J Physiol Heart Circ Physiol. 2010 Apr;298(4):H1146-54. doi: 10.1152/ajpheart.00252.2009. Epub 2010 Feb 5. Am J Physiol Heart Circ Physiol. 2010. PMID: 20139324 Free PMC article.
-
Focal adhesion kinase and Src mediate microvascular hyperpermeability caused by fibrinogen- γC- terminal fragments.PLoS One. 2020 Apr 30;15(4):e0231739. doi: 10.1371/journal.pone.0231739. eCollection 2020. PLoS One. 2020. PMID: 32352989 Free PMC article.
-
Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability.Front Immunol. 2019 May 9;10:1037. doi: 10.3389/fimmu.2019.01037. eCollection 2019. Front Immunol. 2019. PMID: 31143182 Free PMC article. Review.
References
-
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15512. - PubMed
-
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. - PubMed
-
- Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol. 1996;271:H2520–2528. - PubMed
-
- Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2003;39:225–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

