GABA mediates autoreceptor feedback inhibition in the rat carotid body via presynaptic GABAB receptors and TASK-1
- PMID: 12949228
- PMCID: PMC2343478
- DOI: 10.1113/jphysiol.2003.048298
GABA mediates autoreceptor feedback inhibition in the rat carotid body via presynaptic GABAB receptors and TASK-1
Abstract
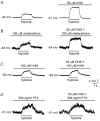
Background K+ channels exert control over neuronal excitability by regulating resting potential and input resistance. Here, we show that GABAB receptor-mediated activation of a background K+ conductance modulates transmission at rat carotid body chemosensory synapses in vitro. Carotid body chemoreceptor (type I) cells expressed GABAB(1) and GABAB(2) subunits as well as endogenous GABA. The GABAB receptor agonist baclofen activated an anandamide- and Ba2+-sensitive TASK-1-like background K+ conductance in chemoreceptor cell clusters, but was without effect on voltage-gated Ca2+ channels. Hydroxysaclofen (50 microM), 5-aminovaleric acid (100 microM) and CGP 55845 (100 nM), selective GABAB receptor blockers, potentiated the hypoxia-induced receptor potential; this effect was abolished by pre-treatment with pertussis toxin (PTX; 500 ng ml-1), an inhibitor of Gi, or by H-89 (50 microM), a selective inhibitor of protein kinase A. The protein kinase C inhibitor chelerythrine chloride (100 microM) was without effect on this potentiation. GABAB receptor blockers also caused depolarisation of type I cells in clusters, and enhanced spike discharge in spontaneously firing cells. In functional co-cultures of type I clusters and petrosal sensory neurones, GABAB receptor blockers potentiated hypoxia-induced postsynaptic chemosensory responses mediated by the fast-acting transmitters ACh and ATP. Thus GABAB receptor-mediated activation of TASK-1 or a related channel provides a presynaptic autoregulatory feedback mechanism that modulates fast synaptic transmission in the rat carotid body.
Figures
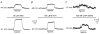
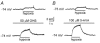

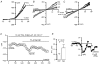

Similar articles
-
Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons.J Neurosci. 1998 Mar 1;18(5):1913-22. doi: 10.1523/JNEUROSCI.18-05-01913.1998. J Neurosci. 1998. PMID: 9465016 Free PMC article.
-
GABAB receptor-mediated responses in GABAergic projection neurones of rat nucleus reticularis thalami in vitro.J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):845-54. doi: 10.1113/jphysiol.1996.sp021427. J Physiol. 1996. PMID: 8799904 Free PMC article.
-
GABAA receptor-mediated IPSCs in rat thalamic sensory nuclei: patterns of discharge and tonic modulation by GABAB autoreceptors.J Physiol. 1997 Jul 1;502 ( Pt 1)(Pt 1):91-104. doi: 10.1111/j.1469-7793.1997.091bl.x. J Physiol. 1997. PMID: 9234199 Free PMC article.
-
A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system.Prog Neurobiol. 1995 Jul;46(4):423-62. doi: 10.1016/0301-0082(95)00012-k. Prog Neurobiol. 1995. PMID: 8532848 Review.
-
The role of GABAB receptors in the subcortical pathways of the mammalian auditory system.Front Endocrinol (Lausanne). 2023 Aug 11;14:1195038. doi: 10.3389/fendo.2023.1195038. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635966 Free PMC article. Review.
Cited by
-
Postsynaptic action of GABA in modulating sensory transmission in co-cultures of rat carotid body via GABA(A) receptors.J Physiol. 2009 Jan 15;587(2):329-44. doi: 10.1113/jphysiol.2008.165035. Epub 2008 Nov 24. J Physiol. 2009. PMID: 19029183 Free PMC article.
-
Cholinergic Chemotransmission and Anesthetic Drug Effects at the Carotid Bodies.Molecules. 2020 Dec 17;25(24):5974. doi: 10.3390/molecules25245974. Molecules. 2020. PMID: 33348537 Free PMC article. Review.
-
Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors.J Physiol. 2014 Aug 15;592(16):3419-26. doi: 10.1113/jphysiol.2013.269829. Epub 2014 Mar 24. J Physiol. 2014. PMID: 24665097 Free PMC article. Review.
-
Protein phosphatase modulation of somatostatin receptor signaling in the mouse hippocampus.Neuropharmacology. 2015 Dec;99:232-41. doi: 10.1016/j.neuropharm.2015.07.004. Epub 2015 Jul 18. Neuropharmacology. 2015. PMID: 26196943 Free PMC article.
-
Role of K₂p channels in stimulus-secretion coupling.Pflugers Arch. 2015 May;467(5):1001-11. doi: 10.1007/s00424-014-1663-3. Epub 2014 Dec 6. Pflugers Arch. 2015. PMID: 25476848 Free PMC article. Review.
References
-
- Bairam A, Neji H, De Grandpre P, Carroll JL. Autoreceptor mechanism regulating carotid body dopamine release from adult and 10-day-old rabbits. Resp Physiol. 2000;120:27–34. - PubMed
-
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurones. Resp Physiol. 2001;129:159–174. - PubMed
-
- Benot AR, Lopez-Barneo J. Feedback inhibition of Ca2+ currents by dopamine in glomus cells of the carotid body. Eur J Neurosci. 1990;2:809–812. - PubMed
-
- Bowery N, Enna SJ. γ-Aminobutyric acidB receptors: first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;2:2–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

