Nitric oxide regulates K+ and Cl- channels in guard cells through a subset of abscisic acid-evoked signaling pathways
- PMID: 12949257
- PMCID: PMC196936
- DOI: 10.1073/pnas.1434381100
Nitric oxide regulates K+ and Cl- channels in guard cells through a subset of abscisic acid-evoked signaling pathways
Abstract
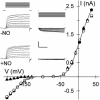
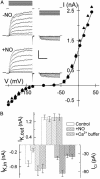
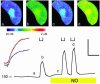
Abscisic acid (ABA) triggers a complex sequence of signaling events that lead to concerted modulation of ion channels at the plasma membrane of guard cells and solute efflux to drive stomatal closure in plant leaves. Recent work has indicated that nitric oxide (NO) and its synthesis are a prerequisite for ABA signal transduction in Arabidopsis and Vicia guard cells. Its mechanism(s) of action is not well defined in guard cells and, generally, in higher plants. Here we show directly that NO selectively regulates Ca2+-sensitive ion channels of Vicia guard cells by promoting Ca2+ release from intracellular stores to raise cytosolic-free [Ca2+]. NO-sensitive Ca2+ release was blocked by antagonists of guanylate cyclase and cyclic ADP ribose-dependent endomembrane Ca2+ channels, implying an action mediated via a cGMP-dependent cascade. NO did not recapitulate ABA-evoked control of plasma membrane Ca2+ channels and Ca2+-insensitive K+ channels, and NO scavengers failed to block the activation of these K+ channels evoked by ABA. These results place NO action firmly within one branch of the Ca2+-signaling pathways engaged by ABA and define the boundaries of parallel signaling events in the control of guard cell movements.
Figures


References
-
- Gibson, A. D. & Garbers, D. L. (2000) Annu. Rev. Neurosci. 23, 417-439. - PubMed
-
- Stamler, J. S. (1994) Cell 78, 931-936. - PubMed
-
- Ahern, G. P., Klyachko, V. A. & Jackson, M. B. (2002) Trends Neurosci. 25, 510-517. - PubMed
-
- Renganathan, M., Cummins, T. R. & Waxman, S. G. (2002) J. Neurophysiol. 87, 761-775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

