Detection and discrimination of orthopoxviruses using microarrays of immobilized oligonucleotides
- PMID: 12951214
- PMCID: PMC9533938
- DOI: 10.1016/s0166-0934(03)00193-9
Detection and discrimination of orthopoxviruses using microarrays of immobilized oligonucleotides
Abstract
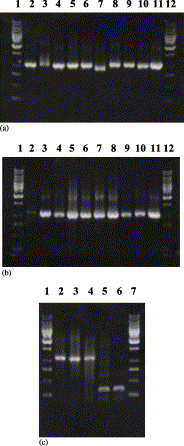
Variola virus (VARV), causing smallpox, is a potential biological weapon. Methods to detect VARV rapidly and to differentiate it from other viruses causing similar clinical syndromes are needed urgently. We have developed a new microarray-based method that detects simultaneously and discriminates four orthopoxvirus (OPV) species pathogenic for humans (variola, monkeypox, cowpox, and vaccinia viruses) and distinguishes them from chickenpox virus (varicella-zoster virus or VZV). The OPV gene C23L/B29R, encoding the CC-chemokine binding protein, was sequenced for 41 strains of seven species of orthopox viruses obtained from different geographical regions. Those C23L/B29R sequences and the ORF 62 sequences from 13 strains of VZV (selected from GenBank) were used to design oligonucleotide probes that were immobilized on an aldehyde-coated glass surface (a total of 57 probes). The microchip contained several unique 13-21 bases long oligonucleotide probes specific to each virus species to ensure redundancy and robustness of the assay. A region approximately 1100 bases long was amplified from samples of viral DNA and fluorescently labeled with Cy5-modified dNTPs, and single-stranded DNA was prepared by strand separation. Hybridization was carried out under plastic coverslips, resulting in a fluorescent pattern that was quantified using a confocal laser scanner. 49 known and blinded samples of OPV DNA, representing different OPV species, and two VZV strains were tested. The oligonucleotide microarray hybridization technique identified reliably and correctly all samples. This new procedure takes only 3 h, and it can be used for parallel testing of multiple samples.
Figures


Similar articles
-
Microarray assay for detection and discrimination of Orthopoxvirus species.J Med Virol. 2006 Oct;78(10):1325-40. doi: 10.1002/jmv.20698. J Med Virol. 2006. PMID: 16927285
-
Real-time PCR to identify variola virus or other human pathogenic orthopox viruses.Clin Chem. 2007 Apr;53(4):606-13. doi: 10.1373/clinchem.2006.068635. Epub 2007 Mar 1. Clin Chem. 2007. PMID: 17332145
-
[Identification of orthopoxvirus species using oligonucleotide micro-chips].Vopr Virusol. 2003 Jan-Feb;48(1):4-9. Vopr Virusol. 2003. PMID: 12608052 Russian.
-
The effective factors in human-specific tropism and viral pathogenicity in orthopoxviruses.Cell Biol Int. 2023 Feb;47(2):341-351. doi: 10.1002/cbin.11941. Epub 2022 Nov 1. Cell Biol Int. 2023. PMID: 36317465 Review.
-
Orthopoxvirus diagnostics.Methods Mol Biol. 2004;269:119-34. doi: 10.1385/1-59259-789-0:119. Methods Mol Biol. 2004. PMID: 15114012 Review.
Cited by
-
Diagnostic approaches for viruses and prions in stem cell banks.Virology. 2006 Mar 30;347(1):1-10. doi: 10.1016/j.virol.2005.11.026. Epub 2005 Dec 27. Virology. 2006. PMID: 16380145 Free PMC article. Review.
-
A genomics-based approach to biodefence preparedness.Nat Rev Genet. 2004 Jan;5(1):23-33. doi: 10.1038/nrg1245. Nat Rev Genet. 2004. PMID: 14708013 Free PMC article. Review.
-
Recent advances in the diagnosis monkeypox: implications for public health.Expert Rev Mol Diagn. 2022 Jul;22(7):739-744. doi: 10.1080/14737159.2022.2116979. Epub 2022 Aug 24. Expert Rev Mol Diagn. 2022. PMID: 35997157 Free PMC article.
-
Emerging viral infections.Clin Lab Med. 2004 Sep;24(3):773-95, vii. doi: 10.1016/j.cll.2004.05.002. Clin Lab Med. 2004. PMID: 15325064 Free PMC article. Review.
-
Comprehensive viral oligonucleotide probe design using conserved protein regions.Nucleic Acids Res. 2008 Jan;36(1):e3. doi: 10.1093/nar/gkm1106. Epub 2007 Dec 13. Nucleic Acids Res. 2008. PMID: 18079152 Free PMC article.
References
-
- Arita M., Tagaya I. Structural polypeptides of several strains of orthopoxvirus. Microbiol. Immunol. 1977;21(6):343–346. - PubMed
-
- Baxby D. Identification and interrelationships of the variola/vaccinia subgroup of poxviruses. Prog. Med. Virol. 1975;19:215–246. - PubMed
-
- Breman J.G., Henderson D.A. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. New Engl. J. Med. 1998;339(8):556–559. - PubMed
-
- Breman J.G., Henderson D.A. Diagnosis and management of smallpox. New Engl. J. Med. 2002;346(17):1300–1308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

