Recent advances in the discovery and delivery of vaccine adjuvants
- PMID: 12951579
- PMCID: PMC7097252
- DOI: 10.1038/nrd1176
Recent advances in the discovery and delivery of vaccine adjuvants
Abstract
Adjuvant design has historically had a touch of alchemy at its heart due to its reliance on the complex biology of innate immune activation. However, a new mechanistic understanding of innate immunity, combined with new adjuvant and delivery platforms for exploiting this knowledge, has led to significant advances recently. Although many challenges remain, the field is moving rapidly and the proper tools and methodologies are in place for the use of traditional drug discovery engines in guiding the development of vaccine adjuvants. In this review, we outline the current trends in immune potentiator, delivery system and adjuvant design that will shape the vaccines of the future.
Figures

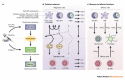


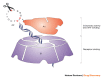
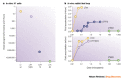
References
-
- WHO World Health Report (2002).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

