Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation
- PMID: 12952183
- PMCID: PMC3564252
Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation
Abstract
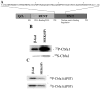
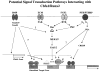
The Cbfa1/Runx2 transcription factor is essential for osteoblast differentiation. However, levels of Runx2 are often not well correlated with its transcriptional activity suggesting that this factor must be activated either by covalent modification or through interactions with other nuclear components. Runx2 is phosphorylated and activated by the mitogen-activated protein kinase (MAPK) pathway. This pathway is stimulated in at least two ways: by binding of type I collagen to alpha2beta1 integrins on the osteoblast surface and by treatment of cells with the osteogenic growth factor, FGF2. Protein kinase A (PKA) also may phosphorylate/activate Runx2 under certain conditions. Runx2 activity also is enhanced by factors known to stimulate specific signal transduction pathways such as PTH/PTHrP (signals through PKA and PKC pathways) and BMPs (Signal through Smad proteins). Interactions with Runx2 are complex involving both binding of distinct components such as AP-1 factors and Smads to separate sites on DNA, direct interactions between Runx2 and AP-1/Smad factors and MAPK or PKA-dependent Runx2 phosphorylation. These findings suggest that Runx2 plays a central role in coordinating multiple signals involved in osteoblast differentiation.
Figures


Similar articles
-
Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways.J Cell Biochem. 2003 Feb 15;88(3):446-54. doi: 10.1002/jcb.10369. J Cell Biochem. 2003. PMID: 12532321 Review.
-
Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway.Exp Cell Res. 2004 Sep 10;299(1):128-36. doi: 10.1016/j.yexcr.2004.05.025. Exp Cell Res. 2004. PMID: 15302580
-
Identification of potential modifiers of Runx2/Cbfa1 activity in C2C12 cells in response to bone morphogenetic protein-7.Cells Tissues Organs. 2004;176(1-3):28-40. doi: 10.1159/000075025. Cells Tissues Organs. 2004. PMID: 14745233
-
Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein.Oncogene. 2002 Oct 17;21(47):7156-63. doi: 10.1038/sj.onc.1205937. Oncogene. 2002. PMID: 12370805
-
Smad-Runx interactions during chondrocyte maturation.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review.
Cited by
-
Intramembranous bone healing process subsequent to tooth extraction in mice: micro-computed tomography, histomorphometric and molecular characterization.PLoS One. 2015 May 29;10(5):e0128021. doi: 10.1371/journal.pone.0128021. eCollection 2015. PLoS One. 2015. PMID: 26023920 Free PMC article.
-
Inhibition of Runx2 signaling by TNF-α in ST2 murine bone marrow stromal cells undergoing osteogenic differentiation.In Vitro Cell Dev Biol Anim. 2016 Dec;52(10):1026-1033. doi: 10.1007/s11626-016-0068-3. Epub 2016 Jul 11. In Vitro Cell Dev Biol Anim. 2016. PMID: 27401008
-
Control of bone development by P2X and P2Y receptors expressed in mesenchymal and hematopoietic cells.Gene. 2015 Oct 1;570(1):1-7. doi: 10.1016/j.gene.2015.06.031. Epub 2015 Jun 14. Gene. 2015. PMID: 26079571 Free PMC article. Review.
-
Thyroid-Stimulating Hormone Favors Runx2-Mediated Matrix Mineralization in HOS and SaOS2 Cells: An In Vitro and In Silico Approach.Molecules. 2022 Jan 18;27(3):613. doi: 10.3390/molecules27030613. Molecules. 2022. PMID: 35163879 Free PMC article.
-
Cellular and extracellular matrix of bone, with principles of synthesis and dependency of mineral deposition on cell membrane transport.Am J Physiol Cell Physiol. 2020 Jan 1;318(1):C111-C124. doi: 10.1152/ajpcell.00120.2019. Epub 2019 Sep 18. Am J Physiol Cell Physiol. 2020. PMID: 31532718 Free PMC article. Review.
References
-
- Ducy P, Schinke T, Karsenty G. The osteoblast: A sophisticated fibroblast under central surveillance. Science. 2000;289(5484):1501–1504. - PubMed
-
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. - PubMed
-
- Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, Owen MJ, Mertelsmann R, Zabel BU, Olsen BR. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89(5):773–779. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. - PubMed
-
- Kern B, Shen J, Starbuck M, Karsenty G. Cbfa1 contributes to the osteoblast-specific expression of type I collagen genes. J Biol Chem. 2000;5:5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
