Differential requirements for AP-2 in clathrin-mediated endocytosis
- PMID: 12952931
- PMCID: PMC2172816
- DOI: 10.1083/jcb.200304069
Differential requirements for AP-2 in clathrin-mediated endocytosis
Abstract
AP-2 complexes are key components in clathrin-mediated endocytosis (CME). They trigger clathrin assembly, interact directly with cargo molecules, and recruit a number of endocytic accessory factors. Adaptor-associated kinase (AAK1), an AP-2 binding partner, modulates AP-2 function by phosphorylating its mu2 subunit. Here, we examined the effects of adenoviral-mediated overexpression of WT AAK1, kinase-dead, and truncation mutants in HeLa cells, and show that AAK1 also regulates AP-2 function in vivo. WT AAK1 overexpression selectively blocks transferrin (Tfn) receptor and LRP endocytosis. Inhibition was kinase independent, but required the full-length AAK1 as truncation mutants were not inhibitory. Although changes in mu2 phosphorylation were not detected, AAK1 overexpression significantly decreased the phosphorylation of large adaptin subunits and the normally punctate AP-2 distribution was dispersed, suggesting that AAK1 overexpression inhibited Tfn endocytosis by functionally sequestering AP-2. Surprisingly, clathrin distribution and EGF uptake were unaffected by AAK1 overexpression. Thus, AP-2 may not be stoichiometrically required for coat assembly, and may have a more cargo-selective function in CME than previously thought.
Figures
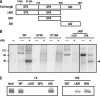

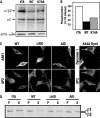
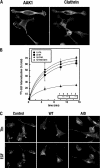
References
-
- Brodsky, F.M., C.Y. Chen, C. Knuehl, M.C. Towler, and D.E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517–568. - PubMed
-
- Collins, B.M., A.J. McCoy, H.M. Kent, P.R. Evans, and D.J. Owen. 2002. Molecular architecture and functional model of the endocytic AP-2 complex. Cell. 109:523–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

