GPI-anchored uPAR requires Endo180 for rapid directional sensing during chemotaxis
- PMID: 12952933
- PMCID: PMC2172817
- DOI: 10.1083/jcb.200302124
GPI-anchored uPAR requires Endo180 for rapid directional sensing during chemotaxis
Abstract
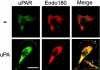
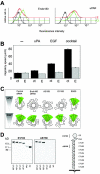
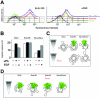
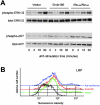
Urokinase-type plasminogen activator (uPA) and its receptor (uPAR) play an important role in cell guidance and chemotaxis during normal and pathological events. uPAR is GPI-anchored and the mechanism by which it transmits intracellular polarity cues across the plasma membrane during directional sensing has not been elucidated. The constitutively recycling endocytic receptor Endo180 forms a trimolecular complex with uPAR in the presence of uPA, hence its alternate name uPAR-associated protein. Here, we demonstrate that Endo180 is a general promoter of random cell migration and has a more specific function in cell chemotaxis up a uPA gradient. Endo180 expression was demonstrated to enhance uPA-mediated filopodia production and promote rapid activation of Cdc42 and Rac. Expression of a noninternalizing Endo180 mutant revealed that promotion of random cell migration requires receptor endocytosis, whereas the chemotactic response to uPA does not. From these studies, we conclude that Endo180 is a crucial link between uPA-uPAR and setting of the internal cellular compass.
Figures

References
-
- Baggiolini, M. 2001. Chemokines in pathology and medicine. J. Intern. Med. 250:91–104. - PubMed
-
- Behrendt, N., O.N. Jensen, L.H. Engelholm, E. Mortz, M. Mann, and K. Dano. 2000. A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem. 275:1993–2002. - PubMed
-
- Blasi, F. 1993. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 15:105–111. - PubMed
-
- Blasi, F., and P. Carmeliet. 2002. uPAR: A versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 3:932–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

