Clathrin-mediated endocytosis in AP-2-depleted cells
- PMID: 12952941
- PMCID: PMC2172830
- DOI: 10.1083/jcb.200305145
Clathrin-mediated endocytosis in AP-2-depleted cells
Abstract
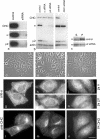
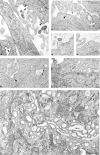
We have used RNA interference to knock down the AP-2 mu2 subunit and clathrin heavy chain to undetectable levels in HeLaM cells. Clathrin-coated pits associated with the plasma membrane were still present in the AP-2-depleted cells, but they were 12-fold less abundant than in control cells. No clathrin-coated pits or vesicles could be detected in the clathrin-depleted cells, and post-Golgi membrane compartments were swollen. Receptor-mediated endocytosis of transferrin was severely inhibited in both clathrin- and AP-2-depleted cells. Endocytosis of EGF, and of an LDL receptor chimera, were also inhibited in the clathrin-depleted cells; however, both were internalized as efficiently in the AP-2-depleted cells as in control cells. These results indicate that AP-2 is not essential for clathrin-coated vesicle formation at the plasma membrane, but that it is one of several endocytic adaptors required for the uptake of certain cargo proteins including the transferrin receptor. Uptake of the EGF and LDL receptors may be facilitated by alternative adaptors.
Figures




References
-
- Aguilar, R.C., H.A. Watson, and B. Wendland. 2003. The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278:10737–10743. - PubMed
-
- Boll, W., I. Rapoport, C. Brunner, Y. Modis, S. Prehn, and T. Kirchhausen. 2002. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppΦ sorting signals at distinct sites. Traffic. 3:590–600. - PubMed
-
- Bonifacino, J.S., and L.M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. In press. - PubMed
-
- Chang, C.P., C.S. Lazar, B.J. Walsh, M. Komuro, J.F. Collawn, L.A. Kuhn, J.A. Tainer, I.S. Trowbridge, M.G. Farquhar, M.G. Rosenfeld, et al. 1993. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268:19312–19320. - PubMed
-
- Conner, S.D., and S.L. Schmid. 2003. a. Regulated portals of entry into the cell. Nature. 422:37–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

