Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis
- PMID: 12952943
- PMCID: PMC2172815
- DOI: 10.1083/jcb.200304105
Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis
Abstract
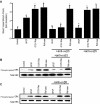
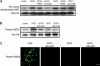
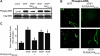
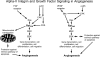
Antagonists of alphavbeta3 and alphavbeta5 disrupt angiogenesis in response to bFGF and VEGF, respectively. Here, we show that these alphav integrins differentially contribute to sustained Ras-extracellular signal-related kinase (Ras-ERK) signaling in blood vessels, a requirement for endothelial cell survival and angiogenesis. Inhibition of FAK or alphavbeta5 disrupted VEGF-mediated Ras and c-Raf activity on the chick chorioallantoic membrane, whereas blockade of FAK or integrin alphavbeta3 had no effect on bFGF-mediated Ras activity, but did suppress c-Raf activation. Furthermore, retroviral delivery of active Ras or c-Raf promoted ERK activity and angiogenesis, which anti-alphavbeta5 blocked upstream of Ras, whereas anti-alphavbeta3 blocked downstream of Ras, but upstream of c-Raf. The activation of c-Raf by bFGF/alphavbeta3 not only depended on FAK, but also required p21-activated kinase-dependent phosphorylation of serine 338 on c-Raf, whereas VEGF-mediated c-Raf phosphorylation/activation depended on Src, but not Pak. Thus, integrins alphavbeta3 and alphavbeta5 differentially regulate the Ras-ERK pathway, accounting for distinct vascular responses during two pathways of angiogenesis.
Figures



References
-
- Alavi, A., J.D. Hood, R. Frausto, D.G. Stupack, and D.A. Cheresh. 2003. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 301:94–96. - PubMed
-
- Alon, T., I. Hemo, A. Itin, J. Pe'er, J. Stone, and E. Keshet. 1995. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1:1024–1028. - PubMed
-
- Aplin, A.E., A.K. Howe, and R.L. Juliano. 1999. Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell Biol. 11:737–744. - PubMed
-
- Barberis, L., K.K. Wary, G. Fiucci, F. Liu, E. Hirsch, M. Brancaccio, F. Altruda, G. Tarone, and F.G. Giancotti. 2000. Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem. 275:36532–36540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

