The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size
- PMID: 12953103
- PMCID: PMC181323
- DOI: 10.1105/tpc.013557
The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size
Abstract
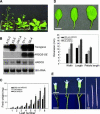
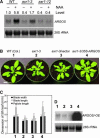
During plant development, the final size of an organ is regulated and determined by various developmental signals; however, the molecular mechanisms by which these signals are transduced and the mediators involved are largely unknown. Here, we show that ARGOS, a novel Arabidopsis gene that is highly induced by auxin, is involved in organ size control. Transgenic plants expressing sense or antisense ARGOS cDNA display enlarged or reduced aerial organs, respectively. The alteration in organ size is attributable mainly to changes in cell number and the duration of organ growth. Ectopic expression of ARGOS prolongs the expression of AINTEGUMENTA (ANT) and CycD3;1 as well as the neoplastic activity of leaf cells. Moreover, organ enlargement in plants overexpressing ARGOS can be blocked by the loss of function of ANT, implying that ARGOS functions upstream of ANT to affect the meristematic competence of organ cells. The induction of ARGOS by auxin is attenuated or abolished in auxin-resistant1 (axr1), and overexpression of ARGOS partially restores axr1 organ development. These results suggest that ARGOS may transduce auxin signals downstream of AXR1 to regulate cell proliferation and organ growth through ANT during organogenesis.
Figures




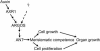
References
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of Arabidopsis thaliana plants. C. R. Acad. Sci. Paris 316, 1194–1199.
-
- Beemster, G.T.S., Fiorani, F., and Inzé, D. (2003). Cell cycle: The key to plant growth control? Trends Plant Sci. 8, 154–158. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

