Crystal structure of a wild-type Cre recombinase-loxP synapse reveals a novel spacer conformation suggesting an alternative mechanism for DNA cleavage activation
- PMID: 12954782
- PMCID: PMC203317
- DOI: 10.1093/nar/gkg732
Crystal structure of a wild-type Cre recombinase-loxP synapse reveals a novel spacer conformation suggesting an alternative mechanism for DNA cleavage activation
Abstract
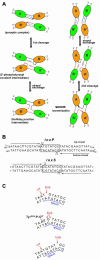
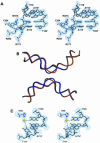
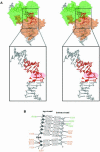
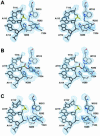
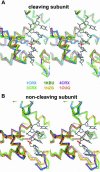
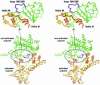
Escherichia coli phage P1 Cre recombinase catalyzes the site-specific recombination of DNA containing loxP sites. We report here two crystal structures of a wild-type Cre recombinase-loxP synaptic complex corresponding to two distinct reaction states: an initial pre-cleavage complex, trapped using a phosphorothioate modification at the cleavable scissile bond that prevents the recombination reaction, and a 3'-phosphotyrosine protein-DNA intermediate resulting from the first strand cleavage. In contrast to previously determined Cre complexes, both structures contain a full tetrameric complex in the asymmetric unit, unequivocally showing that the anti-parallel arrangement of the loxP sites is an intrinsic property of the Cre-loxP recombination synapse. The conformation of the spacer is different to the one observed for the symmetrized loxS site: a kink next to the scissile phosphate in the top strand of the pre-cleavage complex leads to unstacking of the TpG step and a widening of the minor groove. This side of the spacer is interacting with a 'cleavage-competent' Cre subunit, suggesting that the first cleavage occurs at the ApT step in the top strand. This is further confirmed by the structure of the 3'-phosphotyrosine intermediate, where the DNA is cleaved in the top strands and covalently linked to the 'cleavage-competent' subunits. The cleavage is followed by a movement of the C-terminal part containing the attacking Y324 and the helix N interacting with the 'non-cleaving' subunit. This rearrangement could be responsible for the interconversion of Cre subunits. Our results also suggest that the Cre-induced kink next to the scissile phosphodiester activates the DNA for cleavage at this position and facilitates strand transfer.
Figures


Similar articles
-
The order of strand exchanges in Cre-LoxP recombination and its basis suggested by the crystal structure of a Cre-LoxP Holliday junction complex.J Mol Biol. 2002 May 24;319(1):107-27. doi: 10.1016/S0022-2836(02)00246-2. J Mol Biol. 2002. PMID: 12051940 Free PMC article.
-
Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse.Nature. 1997 Sep 4;389(6646):40-6. doi: 10.1038/37925. Nature. 1997. PMID: 9288963
-
Sequence of the loxP site determines the order of strand exchange by the Cre recombinase.J Mol Biol. 2003 Feb 14;326(2):397-412. doi: 10.1016/s0022-2836(02)01429-8. J Mol Biol. 2003. PMID: 12559909
-
A structural view of cre-loxp site-specific recombination.Annu Rev Biophys Biomol Struct. 2001;30:87-104. doi: 10.1146/annurev.biophys.30.1.87. Annu Rev Biophys Biomol Struct. 2001. PMID: 11340053 Review.
-
Dynamics in Cre-loxP site-specific recombination.Curr Opin Struct Biol. 2024 Oct;88:102878. doi: 10.1016/j.sbi.2024.102878. Epub 2024 Jul 18. Curr Opin Struct Biol. 2024. PMID: 39029281 Free PMC article. Review.
Cited by
-
Real-time single-molecule tethered particle motion analysis reveals mechanistic similarities and contrasts of Flp site-specific recombinase with Cre and λ Int.Nucleic Acids Res. 2013 Aug;41(14):7031-47. doi: 10.1093/nar/gkt424. Epub 2013 Jun 3. Nucleic Acids Res. 2013. PMID: 23737451 Free PMC article.
-
Mechanisms of Cre recombinase synaptic complex assembly and activation illuminated by Cryo-EM.Nucleic Acids Res. 2022 Feb 22;50(3):1753-1769. doi: 10.1093/nar/gkac032. Nucleic Acids Res. 2022. PMID: 35104890 Free PMC article.
-
Structural and functional characterization of MrpR, the master repressor of the Bacillus subtilis prophage SPβ.Nucleic Acids Res. 2023 Sep 22;51(17):9452-9474. doi: 10.1093/nar/gkad675. Nucleic Acids Res. 2023. PMID: 37602373 Free PMC article.
-
A Cre Transcription Fidelity Reporter Identifies GreA as a Major RNA Proofreading Factor in Escherichia coli.Genetics. 2017 May;206(1):179-187. doi: 10.1534/genetics.116.198960. Epub 2017 Mar 24. Genetics. 2017. PMID: 28341651 Free PMC article.
-
Engineering spacer specificity of the Cre/loxP system.Nucleic Acids Res. 2024 Jul 22;52(13):8017-8031. doi: 10.1093/nar/gkae481. Nucleic Acids Res. 2024. PMID: 38869070 Free PMC article.
References
-
- Van Duyne G.D. (2001) A structural view of cre-loxp site-specific recombination. Annu. Rev. Biophys Biomol. Struct., 30, 87–104. - PubMed
-
- Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science, 265, 103–106. - PubMed
-
- Yu Y. and Bradley,A. (2001) Engineering chromosomal rearrangements in mice. Nat. Rev. Genet., 2, 780–790. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

