Ketamine-xylazine-induced slow (< 1.5 Hz) oscillations in the rat piriform (olfactory) cortex are functionally correlated with respiration
- PMID: 12954860
- PMCID: PMC6740491
- DOI: 10.1523/JNEUROSCI.23-22-07993.2003
Ketamine-xylazine-induced slow (< 1.5 Hz) oscillations in the rat piriform (olfactory) cortex are functionally correlated with respiration
Abstract
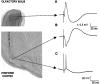
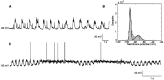
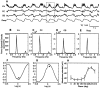
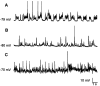
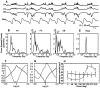
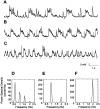
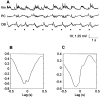
The occurrence of low frequency (<1.5 Hz) cerebral cortical oscillations during slow-wave sleep has recently lead to the suggestion that this pattern of activity is specifically associated with conditions in which the brain is mostly closed to external inputs and running on its own. In the current experiments, we used a combination of in vivo intracellular and extracellular field potential recordings obtained under conditions of ketamine-xylazine anesthesia to examine slow-wave behavior in the olfactory system. We demonstrate the occurrence of low-frequency oscillations in field potentials of both the olfactory bulb and cortex and in the membrane potentials of cortical pyramidal cells. By monitoring ongoing breathing, we also show that these oscillations are all correlated with the natural breathing cycle. Using a tracheotomized preparation, we demonstrate that slow oscillatory patterns could occasionally be produced even when air is no longer entering the nose, supporting the view that the olfactory system has an intrinsic propensity to oscillate. However, in the case of tracheotomized rats, the amplitude and regularity of the oscillations as well as their patterns of correlation are disrupted. All temporal relationships were restored when air was pulsed into the nostrils. We conclude that, in the olfactory system of freely breathing rats, there is a strong relationship between the occurrence and timing of slow oscillations and the ongoing periodic sensory input resulting from respiration. This coupling between olfactory cortex slow oscillations and respiration may result from the interaction between respiratory-related rhythmic input and the tendency for olfactory structures to oscillate intrinsically. We believe this finding has important functional as well as evolutionary implications.
Figures
References
-
- Achermann P, Borbély AA ( 1997) Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81: 213-222. - PubMed
-
- Amzica F, Steriade M ( 1997) The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology 49: 952-959. - PubMed
-
- Bhalla US, Bower JM ( 1997) Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties. J Comput Neurosci 4: 221-256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
