Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit
- PMID: 12954879
- PMCID: PMC6740486
- DOI: 10.1523/JNEUROSCI.23-22-08159.2003
Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit
Abstract
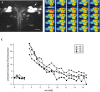
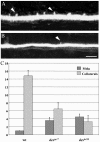
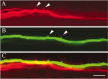
The relatively simple neural circuit driving the escape response in zebrafish offers an excellent opportunity to study properties of neural circuit formation. The hindbrain Mauthner cell is an essential component of this circuit. Mutations in the zebrafish deadly seven/notch1a (des) gene result in supernumerary Mauthner cells. We addressed whether and how these extra cells are incorporated into the escape-response circuit. Calcium imaging revealed that all Mauthner cells in desb420 mutants were active during an elicited escape response. However, the kinematic performance of the escape response in mutant larvae was very similar to wild-type fish. Analysis of the relationship between Mauthner axon collaterals and spinal neurons revealed that there was a decrease in the number of axon collaterals per Mauthner axon in mutant larvae compared with wild-type larvae, indicative of a decrease in the number of synapses formed with target spinal neurons. Moreover, we show that Mauthner axons projecting on the same side of the nervous system have primarily nonoverlapping collaterals. These data support the hypothesis that excess Mauthner cells are incorporated into the escape-response circuit, but they divide their target territory to maintain a normal response, thus demonstrating plasticity in the formation of the escape-response circuit. Such plasticity may be key to the evolution of the startle responses in mammals, which use larger populations of neurons in circuits similar to those in the fish escape response.
Figures

Similar articles
-
Behavioral Role of the Reciprocal Inhibition between a Pair of Mauthner Cells during Fast Escapes in Zebrafish.J Neurosci. 2019 Feb 13;39(7):1182-1194. doi: 10.1523/JNEUROSCI.1964-18.2018. Epub 2018 Dec 21. J Neurosci. 2019. PMID: 30578342 Free PMC article.
-
Celsr3 drives development and connectivity of the acoustic startle hindbrain circuit.PLoS Genet. 2024 Oct 21;20(10):e1011415. doi: 10.1371/journal.pgen.1011415. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39432544 Free PMC article.
-
Evidence for a widespread brain stem escape network in larval zebrafish.J Neurophysiol. 2002 Jan;87(1):608-14. doi: 10.1152/jn.00596.2001. J Neurophysiol. 2002. PMID: 11784774
-
The Mauthner cell and other identified neurons of the brainstem escape network of fish.Prog Neurobiol. 2001 Mar;63(4):467-85. doi: 10.1016/s0301-0082(00)00047-2. Prog Neurobiol. 2001. PMID: 11163687 Review.
-
Spinal network of the Mauthner cell.Brain Behav Evol. 1991;37(5):298-316. doi: 10.1159/000114367. Brain Behav Evol. 1991. PMID: 1933252 Review.
Cited by
-
Brain evolution by brain pathway duplication.Philos Trans R Soc Lond B Biol Sci. 2015 Dec 19;370(1684):20150056. doi: 10.1098/rstb.2015.0056. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26554045 Free PMC article. Review.
-
An immunochemical marker for goldfish Mauthner cells.J Neurosci Methods. 2008 Oct 30;175(1):64-9. doi: 10.1016/j.jneumeth.2008.08.009. Epub 2008 Aug 14. J Neurosci Methods. 2008. PMID: 18771692 Free PMC article.
-
Ear manipulations reveal a critical period for survival and dendritic development at the single-cell level in Mauthner neurons.Dev Neurobiol. 2015 Dec;75(12):1339-51. doi: 10.1002/dneu.22287. Epub 2015 Mar 20. Dev Neurobiol. 2015. PMID: 25787878 Free PMC article.
-
Covariation of brain and skull shapes as a model to understand the role of crosstalk in development and evolution.Evol Dev. 2023 Jan;25(1):85-102. doi: 10.1111/ede.12421. Epub 2022 Nov 14. Evol Dev. 2023. PMID: 36377237 Free PMC article.
-
Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity.J Exp Biol. 2019 Apr 15;222(Pt 8):jeb190058. doi: 10.1242/jeb.190058. J Exp Biol. 2019. PMID: 30988051 Free PMC article. Review.
References
-
- Eaton RC, Lavender WA, Wieland CM ( 1981) Identification of Mauthner-initiated response patterns in goldfish: evidence from simultaneous cinematography and electrophysiology. J Comp Physiol 144: 521-531.
-
- Faber DS, Korn H ( 1978) Electrophysiology of the Mauthner cell: basic properties, synaptic mechanisms, and associated networks. In: Neurobiology of the Mauthner cell. New York: Raven.
-
- Fetcho JR, O'Malley DM ( 1995) Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophys 73: 399-406. - PubMed
-
- Gahtan E, O'Malley D ( 2003) Visually guided injection of identified reticulospinal neurons in zebrafish: a survey of spinal arborization patterns. J Comp Neurol 459: 186-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
