Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo
- PMID: 12954881
- PMCID: PMC6740498
- DOI: 10.1523/JNEUROSCI.23-22-08176.2003
Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo
Abstract
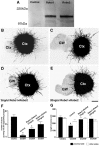
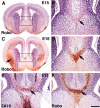
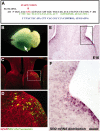
Commissural axons generally cross the midline only once. In the Drosophila nerve cord and mouse spinal cord, commissural axons are guided by Slit only after they cross the midline, where Slit prevents these axons from recrossing the midline. In the developing corpus callosum, Slit2 expressed by the glial wedge guides callosal axons before they cross the midline, as they approach the corticoseptal boundary. These data highlighted a potential difference between the role of Slit2 in guiding commissural axons in the brain compared with the spinal cord. Here, we investigate whether Slit2 also guides callosal axons after they cross the midline. Because such questions cannot be addressed in conventional gene knock-out animals, we used in utero injections of antisense oligonucleotides to specifically deplete Slit2 on only one side of the brain. We used this technique together with a novel in vitro assay of hemisected brain slices to specifically analyze postcrossing callosal axons. We find that in the brain, unlike the spinal cord, Slit2 mediates both precrossing and postcrossing axonal guidance. Depletion of Slit2 on one side of the brain causes axons to defasciculate and, in some cases, to aberrantly enter the septum. Because these axons do not recross the midline, we conclude that the principle function of Slit2 at the cortical midline may be to channel the axons along the correct path and possibly repel them away from the midline. We find no evidence that Slit2 prevents axons from recrossing the midline in the brain.
Figures




Similar articles
-
Multiple Slits regulate the development of midline glial populations and the corpus callosum.Dev Biol. 2012 May 1;365(1):36-49. doi: 10.1016/j.ydbio.2012.02.004. Epub 2012 Feb 11. Dev Biol. 2012. PMID: 22349628
-
Cortical axon guidance by the glial wedge during the development of the corpus callosum.J Neurosci. 2001 Apr 15;21(8):2749-58. doi: 10.1523/JNEUROSCI.21-08-02749.2001. J Neurosci. 2001. PMID: 11306627 Free PMC article.
-
Netrin-DCC signaling regulates corpus callosum formation through attraction of pioneering axons and by modulating Slit2-mediated repulsion.Cereb Cortex. 2014 May;24(5):1138-51. doi: 10.1093/cercor/bhs395. Epub 2013 Jan 9. Cereb Cortex. 2014. PMID: 23302812
-
Glia-neuron interactions at the midline of the developing mammalian brain and spinal cord.Perspect Dev Neurobiol. 1993;1(4):227-36. Perspect Dev Neurobiol. 1993. PMID: 8087547 Review.
-
Slits and their receptors.Adv Exp Med Biol. 2007;621:65-80. doi: 10.1007/978-0-387-76715-4_5. Adv Exp Med Biol. 2007. PMID: 18269211 Review.
Cited by
-
Human ROBO1 regulates white matter structure in corpus callosum.Brain Struct Funct. 2017 Mar;222(2):707-716. doi: 10.1007/s00429-016-1240-y. Epub 2016 May 30. Brain Struct Funct. 2017. PMID: 27240594 Free PMC article.
-
Molecular mechanisms of corpus callosum development: a four-step journey.Front Neuroanat. 2024 Jan 17;17:1276325. doi: 10.3389/fnana.2023.1276325. eCollection 2023. Front Neuroanat. 2024. PMID: 38298831 Free PMC article. Review.
-
A selective defect in the glial wedge as part of the neuroepithelium disruption in hydrocephalus development in the mouse hyh model is associated with complete corpus callosum dysgenesis.Front Cell Neurosci. 2024 Feb 21;18:1330412. doi: 10.3389/fncel.2024.1330412. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38450283 Free PMC article.
-
Astrocyte development and heterogeneity.Cold Spring Harb Perspect Biol. 2014 Nov 20;7(1):a020362. doi: 10.1101/cshperspect.a020362. Cold Spring Harb Perspect Biol. 2014. PMID: 25414368 Free PMC article. Review.
-
The Slit-Robo signalling pathway in nervous system development: a comparative perspective from vertebrates and invertebrates.Open Biol. 2025 Jul;15(7):250026. doi: 10.1098/rsob.250026. Epub 2025 Jul 9. Open Biol. 2025. PMID: 40628293 Free PMC article. Review.
References
-
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JLR, Tessier-Lavigne M (2002) Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33:233-248. - PubMed
-
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T (1999) Slit proteins bind Robo receptors and have an evolutionary conserved role in repulsive axon guidance. Cell 96:795-806. - PubMed
-
- Cummings DM, Malun D, Brunjes PC (1997) Development of the anterior commissure in the opossum: midline extracellular space and glia coincide with early axon decussation. J Neurobiol 32:403-414. - PubMed
-
- Goodhill GJ (2003) A theoretical model of axon guidance by the Robo code. Neural Comput 15:549-564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
