Specificity in the activation and control of transcription factor E2F-dependent apoptosis
- PMID: 12954980
- PMCID: PMC196891
- DOI: 10.1073/pnas.1831408100
Specificity in the activation and control of transcription factor E2F-dependent apoptosis
Abstract
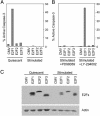
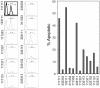
Previous work has demonstrated a role for the E2F1 gene product in signaling apoptosis, both as a result of the deregulation of the Rb/E2F pathway as well as in response to DNA damage. We now show that the ability of cells to suppress the apoptotic potential of E2F1, as might occur during the course of normal cellular proliferation, requires the action of the Ras-phosphoinositide 3-kinase-Akt signaling pathway. In addition, we also identify a domain within the E2F1 protein, previously termed the marked-box domain, that is essential for the apoptotic activity of E2F1 and that distinguishes the E2F1 protein from E2F3. We also show that the E2F1-marked-box domain is essential for the induction of both p53 and p73 accumulation. Importantly, a role for the marked-box domain in the specificity of E2F1-mediated apoptosis coincides with recent work demonstrating a role for this domain in achieving specificity in the activation of transcription. We conclude that the unique capacity of E2F1 to trigger apoptosis reflects a specificity of transcriptional activation potential, and that this role for E2F1 is regulated through the action of the Akt protein kinase.
Figures



References
-
- Dyson, N. (1998) Genes Dev. 12, 2245–2262. - PubMed
-
- Nevins, J. R. (1998) Cell Growth Differ. 9, 585–593. - PubMed
-
- Humbert, P. O., Rogers, C., Ganiatsas, S., Landsberg, R. L., Trimarchi, J. M., Dandapani, S., Brugnara, C., Erdman, S., Schrenzel, M., Bronson, R. T., et al. (2000) Mol. Cell 6, 281–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

