The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes
- PMID: 12954981
- PMCID: PMC196879
- DOI: 10.1073/pnas.1831718100
The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes
Abstract
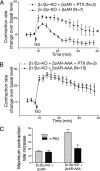
beta1- and beta2-adrenergic receptors (AR) regulate cardiac myocyte function through distinct signaling pathways. In addition to regulating cardiac rate and contractility, beta1AR and beta2AR may play different roles in the pathogenesis of heart failure. Studies on neonatal cardiac myocytes from beta1AR and beta2AR knockout mice suggest that subtype-specific signaling is determined by subtype-specific membrane targeting and trafficking. Stimulation of beta2ARs has a biphasic effect on contraction rate, with an initial increase followed by a sustained Gi-dependent decrease. Recent studies show that a PDZ domain-binding motif at the carboxyl terminus of human beta2AR interacts with ezrin-binding protein 50/sodium-hydrogen exchanger regulatory factor, a PDZ-domain-containing protein. The human beta2AR carboxyl terminus also binds to N-ethylmaleimide-sensitive factor, which does not contain a PDZ domain. We found that mutation of the three carboxyl-terminal amino acids in the mouse beta2AR (beta2AR-AAA) disrupts recycling of the receptor after agonist-induced internalization in cardiac myocytes. Nevertheless, stimulation of the beta2AR-AAA produced a greater contraction rate increase than that of the wild-type beta2AR. This enhanced stimulation of contraction rate can be attributed in part to the failure of the beta2AR-AAA to couple to Gi. We also observed that coupling of endogenous, wild-type beta2AR to Gi in beta1AR knockout myocytes is inhibited by treatment with a membrane-permeable peptide representing the beta2AR carboxyl terminus. These studies demonstrate that association of the carboxyl terminus of the beta2AR with ezrin-binding protein 50/sodium-hydrogen exchanger regulatory factor, N-ethylmaleimide-sensitive factor, or some related proteins dictates physiologic signaling specificity and trafficking in cardiac myocytes.
Figures



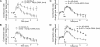
References
-
- Koch, W. J., Milano, C. A. & Lefkowitz, R. J. (1996) Circ. Res. 78, 511–516. - PubMed
-
- Lefkowitz, R. J., Rockman, H. A. & Koch, W. J. (2000) Circulation 101, 1634–1637. - PubMed
-
- Gauthier, C., Langin, D. & Balligand, J. L. (2000) Trends Pharmacol. Sci. 21, 426–431. - PubMed
-
- Devic, E., Xiang, Y., Gould, D. & Kobilka, B. (2001) Mol. Pharmacol. 60, 577–583. - PubMed
-
- Chruscinski, A. J., Rohrer, D. K., Schauble, E., Desai, K. H., Bernstein, D. & Kobilka, B. K. (1999) J. Biol. Chem. 274, 16694–16700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

