Effects of the estrous cycle, pregnancy and interferon tau on expression of cyclooxygenase two (COX-2) in ovine endometrium
- PMID: 12956885
- PMCID: PMC194659
- DOI: 10.1186/1477-7827-1-58
Effects of the estrous cycle, pregnancy and interferon tau on expression of cyclooxygenase two (COX-2) in ovine endometrium
Abstract
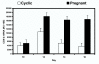
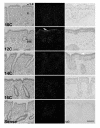
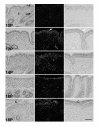
In sheep, the uterus produces luteolytic pulses of prostaglandin F2alpha (PGF) on Days 15 to 16 of estrous cycle to regress the corpus luteum (CL). These PGF pulses are produced by the endometrial lumenal epithelium (LE) and superficial ductal glandular epithelium (sGE) in response to binding of pituitary and/or luteal oxytocin to oxytocin receptors (OTR) and liberation of arachidonic acid, the precursor of PGF. Cyclooxygenase-one (COX-1) and COX-2 are rate-limiting enzymes in PGF synthesis, and COX-2 is the major form expressed in ovine endometrium. During pregnancy recognition, interferon tau (IFNtau), produced by the conceptus trophectoderm, acts in a paracrine manner to suppress development of the endometrial epithelial luteolytic mechanism by inhibiting transcription of estrogen receptor alpha (ERalpha) (directly) and OTR (indirectly) genes. Conflicting studies indicate that IFNtau increases, decreases or has no effect on COX-2 expression in bovine and ovine endometrial cells. In Study One, COX-2 mRNA and protein were detected solely in endometrial LE and sGE of both cyclic and pregnant ewes. During the estrous cycle, COX-2 expression increased from Days 10 to 12 and then decreased to Day 16. During early pregnancy, COX-2 expression increased from Days 10 to 12 and remained higher than in cyclic ewes. In Study Two, intrauterine infusion of recombinant ovine IFNtau in cyclic ewes from Days 11 to 16 post-estrus did not affect COX-2 expression in the endometrial epithelium. These results clearly indicate that IFNtau has no effect on expression of the COX-2 gene in the ovine endometrium. Therefore, antiluteolytic effects of IFNtau are to inhibit ERalpha and OTR gene transcription, thereby preventing endometrial production of luteolytic pulses of PGF. Indeed, expression of COX-2 in the endometrial epithelia as well as conceptus is likely to have a beneficial regulatory role in implantation and development of the conceptus.
Figures

References
-
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine mediated event. Physiol Rev. 1999;79:263–323. - PubMed
-
- Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–98. - PubMed
-
- McCracken JA, Carlson JC, Glew ME, Goding JR, Baird DT, Green K, Samuelsson B. Prostaglandin F2α identified as a luteolytic hormone in sheep. Nature New Biol. 1971;238:129–134. - PubMed
-
- Hooper SB, Watkins WB, Thorburn GD. Oxytocin, oxytocin-associated neurophysin, and prostaglandin F2 alpha concentrations in the utero-ovarian vein of pregnant and nonpregnant sheep. Endocrinology. 1986;119:2590–2597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

