Environmental determinants of Vibrio cholerae biofilm development
- PMID: 12957889
- PMCID: PMC194957
- DOI: 10.1128/AEM.69.9.5079-5088.2003
Environmental determinants of Vibrio cholerae biofilm development
Abstract
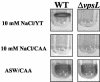
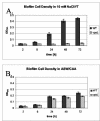
Vibrio cholerae is a versatile bacterium that flourishes in diverse environments, including the human intestine, rivers, lakes, estuaries, and the ocean. Surface attachment is believed to be essential for colonization of all of these natural environments. Previous studies have demonstrated that the vps genes, which encode proteins required for exopolysaccharide synthesis and transport, are required for V. cholerae biofilm development in Luria-Bertani broth. In this work, we showed that V. cholerae forms vps-dependent biofilms and vps-independent biofilms. The vps-dependent and -independent biofilms differ in their environmental activators and in architecture. Our results suggest that environmental activators of vps-dependent biofilm development are present in freshwater, while environmental activators of vps-independent biofilm development are present in seawater. The distinct environmental requirements for the two modes of biofilm development suggest that vps-dependent biofilm development and vps-independent biofilm development may play distinct roles in the natural environment.
Figures







References
-
- Allison, D. G., and M. J. Goldsbrough. 1994. Polysaccharide production in Pseudomonas cepacia. J. Basic Microbiol. 34:3-10. - PubMed
-
- Bechet, M., and R. Blondeau. 2003. Factors associated with the adherence and biofilm formation by Aeromonas caviae on glass surfaces. J. Appl. Microbiol. 94:1072-1078. - PubMed
-
- Burdman, S., E. Jurkevitch, B. Schwartsburd, M. Hampel, and Y. Okon. 1998. Aggregation in Azospirillum brasilense: effects of chemical and physical factors and involvement of extracellular components. Microbiology 144:1989-1999. - PubMed
-
- Colwell, R. R., and W. M. Spira. 1992. The ecology of Vibrio cholerae, p. 107-127. In D. Barua and W. B. I. Greenough (ed.), Cholera. Plenum, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

