Molecular detection and quantitation of the red tide dinoflagellate Karenia brevis in the marine environment
- PMID: 12957971
- PMCID: PMC194946
- DOI: 10.1128/AEM.69.9.5726-5730.2003
Molecular detection and quantitation of the red tide dinoflagellate Karenia brevis in the marine environment
Abstract
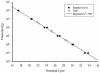
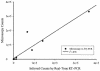
A real-time reverse transcription-PCR method targeting the rbcL gene was developed for the detection and quantitation of the Florida red tide organism, Karenia brevis. The assay was sensitive to less than 1 cell per reaction, did not detect rbcL from 38 nontarget taxa, and accurately quantitated K. brevis organisms in red tide samples from around Florida. These studies have resulted in a sensitive and specific method for K. brevis detection in the marine environment.
Figures

Similar articles
-
Detection and quantification of the red tide dinoflagellate Karenia brevis by real-time nucleic acid sequence-based amplification.Appl Environ Microbiol. 2004 Aug;70(8):4727-32. doi: 10.1128/AEM.70.8.4727-4732.2004. Appl Environ Microbiol. 2004. PMID: 15294808 Free PMC article.
-
Development of a dinoflagellate-oriented PCR primer set leads to detection of picoplanktonic dinoflagellates from Long Island Sound.Appl Environ Microbiol. 2006 Aug;72(8):5626-30. doi: 10.1128/AEM.00586-06. Appl Environ Microbiol. 2006. PMID: 16885319 Free PMC article.
-
Genetic diversity and molecular detection of three toxic dinoflagellate genera (Alexandrium, Dinophysis, and Karenia) from French coasts.Protist. 2002 Sep;153(3):223-38. doi: 10.1078/1434-4610-00100. Protist. 2002. PMID: 12389812
-
Localization of polyketide synthase encoding genes to the toxic dinoflagellate Karenia brevis.Phytochemistry. 2005 Aug;66(15):1767-80. doi: 10.1016/j.phytochem.2005.06.010. Phytochemistry. 2005. PMID: 16051286 Free PMC article.
-
Chimeric plastid proteome in the Florida "red tide" dinoflagellate Karenia brevis.Mol Biol Evol. 2006 Nov;23(11):2026-38. doi: 10.1093/molbev/msl074. Epub 2006 Jul 28. Mol Biol Evol. 2006. PMID: 16877498
Cited by
-
Real-time PCR for detection and quantification of the protistan parasite Perkinsus marinus in environmental waters.Appl Environ Microbiol. 2004 Nov;70(11):6611-8. doi: 10.1128/AEM.70.11.6611-6618.2004. Appl Environ Microbiol. 2004. PMID: 15528525 Free PMC article.
-
Multiple simultaneous detection of Harmful Algal Blooms (HABs) through a high throughput bead array technology, with potential use in phytoplankton community analysis.Harmful Algae. 2009;8(2):196-211. doi: 10.1016/j.hal.2008.05.003. Harmful Algae. 2009. PMID: 20046212 Free PMC article.
-
Quantitative PCR method for enumeration of cells of cryptic species of the toxic marine dinoflagellate Ostreopsis spp. in coastal waters of Japan.PLoS One. 2013 Mar 13;8(3):e57627. doi: 10.1371/journal.pone.0057627. Print 2013. PLoS One. 2013. PMID: 23593102 Free PMC article.
-
Abundance and distribution of Ostreococcus sp. in the San Pedro Channel, California, as revealed by quantitative PCR.Appl Environ Microbiol. 2006 Apr;72(4):2496-506. doi: 10.1128/AEM.72.4.2496-2506.2006. Appl Environ Microbiol. 2006. PMID: 16597949 Free PMC article.
-
Molecular detection of harmful algal blooms (HABs) using locked nucleic acids and bead array technology.Limnol Oceanogr Methods. 2010 Jun 1;8:269-284. doi: 10.4319/lom.2010.8.269. Limnol Oceanogr Methods. 2010. PMID: 21165155 Free PMC article.
References
-
- Aldrich, D. V. 1962. Photoautotrophy in Gymnodinium breve. Science 137:988-990. - PubMed
-
- Geesey, M. E., and P. A. Tester. 1993. Gymnodinium breve: ubiquitous in Gulf of Mexico waters, p. 251-256. In T. J. S. Smayda and Shimizu (ed.), Toxic phytoplankton blooms in the sea: Proceedings of the Fifth International Conference on Toxic Marine Phytoplankton. Elsevier Science Publishing, Inc., New York, N.Y.
-
- Guillard, R. R., and J. H. Ryther. 1962. Studies of marine planktonic diatoms. 1. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8:229-239. - PubMed
-
- Gunther, G., R. H. Williams, C. C. Davis, and F. G. W. Smith. 1947. Catastrophic mass mortality of marine animals and coincident phytoplankton bloom on the west coast of Florida. Ecol. Monogr. 18:311-324.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

