GPI transamidase of Trypanosoma brucei has two previously uncharacterized (trypanosomatid transamidase 1 and 2) and three common subunits
- PMID: 12958211
- PMCID: PMC196864
- DOI: 10.1073/pnas.1833260100
GPI transamidase of Trypanosoma brucei has two previously uncharacterized (trypanosomatid transamidase 1 and 2) and three common subunits
Abstract
Glycosylphosphatidylinositol (GPI) anchor is a membrane attachment mechanism for cell surface proteins widely used in eukaryotes. GPIs are added to proteins posttranslationally by a complex enzyme, GPI transamidase. Previous studies have shown that human and Saccharomyces cerevisiae GPI transamidases are similar and consist of five homologous components: GAA1, GPI8, PIG-S, PIG-T, and PIG-U in humans and Gaa1p, Gpi8p, Gpi17p, Gpi16p, and Cdc91p in S. cerevisiae. We report that GPI transamidase of Trypanosoma brucei (Tb), a causative agent of African sleeping sickness, shares only three components (TbGAA1, TbGPI8, and TbGPI16) with humans and S. cerevisiae but has two other specific components, trypanosomatid transamidase 1 (TTA1) and TTA2. GPI transamidases of both bloodstream form (growing in mammalian blood) and procyclic form (growing in tsetse fly vector) of the parasite have the same five components. Homologues of TTA1 and TTA2 are present in Leishmania and Trypanosoma cruzi but not in mammals, yeasts, flies, nematodes, plants, or malaria parasites, suggesting that these components may play unique roles in attachment of GPI anchors in trypanosomatid parasites and provide good targets for antitrypanosome drugs.
Figures
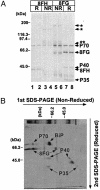


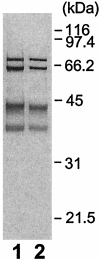
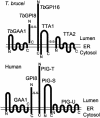
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

