Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures
- PMID: 12958212
- PMCID: PMC196920
- DOI: 10.1073/pnas.1834448100
Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures
Abstract
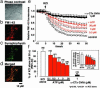
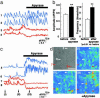
Originally ascribed passive roles in the CNS, astrocytes are now known to have an active role in the regulation of synaptic transmission. Neuronal activity can evoke Ca2+ transients in astrocytes, and Ca2+ transients in astrocytes can evoke changes in neuronal activity. The excitatory neurotransmitter glutamate has been shown to mediate such bidirectional communication between astrocytes and neurons. We demonstrate here that ATP, a primary mediator of intercellular Ca2+ signaling among astrocytes, also mediates intercellular signaling between astrocytes and neurons in hippocampal cultures. Mechanical stimulation of astrocytes evoked Ca2+ waves mediated by the release of ATP and the activation of P2 receptors. Mechanically evoked Ca2+ waves led to decreased excitatory glutamatergic synaptic transmission in an ATP-dependent manner. Exogenous application of ATP does not affect postsynaptic glutamatergic responses but decreased presynaptic exocytotic events. Finally, we show that astrocytes exhibit spontaneous Ca2+ waves mediated by extracellular ATP and that inhibition of these Ca2+ responses enhanced excitatory glutamatergic transmission. We therefore conclude that ATP released from astrocytes exerts tonic and activity-dependent down-regulation of synaptic transmission via presynaptic mechanisms.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

