Nitric oxide inhibits H2O2-induced transferrin receptor-dependent apoptosis in endothelial cells: Role of ubiquitin-proteasome pathway
- PMID: 12958216
- PMCID: PMC196859
- DOI: 10.1073/pnas.1933581100
Nitric oxide inhibits H2O2-induced transferrin receptor-dependent apoptosis in endothelial cells: Role of ubiquitin-proteasome pathway
Abstract
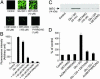
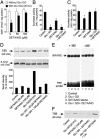
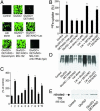
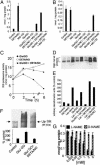
We investigated here the mechanism of cytoprotection of nitric oxide (*NO) in bovine aortic endothelial cells treated with H2O2. NONOates were used as *NO donors that released *NO slowly at a well defined rate in the extracellular and intracellular milieus. H2O2-mediated intracellular dichlorofluorescein fluorescence and apoptosis were enhanced by the transferrin receptor (TfR)-mediated iron uptake. *NO inhibited the TfR-mediated iron uptake, dichlorofluorescein fluorescence, and apoptosis in H2O2-treated cells. *NO increased the proteasomal activity and degradation of nitrated TfR via ubiquitination. Nomega-nitro-L-arginine methyl ester, a nonspecific inhibitor of endogenous *NO biosynthesis, decreased the trypsin-like activity of 26S proteasome. *NO, by activating proteolysis, mitigates TfR-dependent iron uptake, dichlorodihydrofluorescein oxidation, and apoptosis in H2O2-treated bovine aortic endothelial cells. The relevance of biological nitration on redox signaling is discussed.
Figures
Similar articles
-
Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis.Circ Res. 2003 Jan 10;92(1):56-63. doi: 10.1161/01.res.0000048195.15637.ac. Circ Res. 2003. PMID: 12522121
-
Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide.Free Radic Biol Med. 2005 Sep 1;39(5):567-83. doi: 10.1016/j.freeradbiomed.2005.04.016. Free Radic Biol Med. 2005. PMID: 16085176
-
Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells.Free Radic Biol Med. 2006 Mar 15;40(6):1034-44. doi: 10.1016/j.freeradbiomed.2005.10.052. Epub 2005 Nov 17. Free Radic Biol Med. 2006. PMID: 16540399
-
Nitric oxide mitigates peroxide-induced iron-signaling, oxidative damage, and apoptosis in endothelial cells: role of proteasomal function?Arch Biochem Biophys. 2004 Mar 1;423(1):74-80. doi: 10.1016/j.abb.2003.12.037. Arch Biochem Biophys. 2004. PMID: 14989268 Review.
-
The role of the ubiquitin-proteasome pathway in apoptosis.Cell Death Differ. 1999 Apr;6(4):303-13. doi: 10.1038/sj.cdd.4400505. Cell Death Differ. 1999. PMID: 10381632 Review.
Cited by
-
Dual Switch Mechanism of Erythropoietin as an Antiapoptotic and Pro-Angiogenic Determinant in the Retina.ACS Omega. 2020 Aug 12;5(33):21113-21126. doi: 10.1021/acsomega.0c02763. eCollection 2020 Aug 25. ACS Omega. 2020. PMID: 32875248 Free PMC article.
-
Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q.Biophys J. 2009 Feb 18;96(4):1388-98. doi: 10.1016/j.bpj.2008.10.042. Biophys J. 2009. PMID: 19217856 Free PMC article.
-
Distinct Effects of Beta-Amyloid, Its Isomerized and Phosphorylated Forms on the Redox Status and Mitochondrial Functioning of the Blood-Brain Barrier Endothelium.Int J Mol Sci. 2022 Dec 22;24(1):183. doi: 10.3390/ijms24010183. Int J Mol Sci. 2022. PMID: 36613623 Free PMC article.
-
Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis.Sci Rep. 2016 Apr 11;6:24108. doi: 10.1038/srep24108. Sci Rep. 2016. PMID: 27063143 Free PMC article.
-
Oxidants induce alternative splicing of alpha-synuclein: Implications for Parkinson's disease.Free Radic Biol Med. 2010 Feb 1;48(3):377-83. doi: 10.1016/j.freeradbiomed.2009.10.045. Epub 2009 Oct 23. Free Radic Biol Med. 2010. PMID: 19857570 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

