Isolation of an Anaplasma sp. organism from white-tailed deer by tick cell culture
- PMID: 12958265
- PMCID: PMC193820
- DOI: 10.1128/JCM.41.9.4328-4335.2003
Isolation of an Anaplasma sp. organism from white-tailed deer by tick cell culture
Abstract
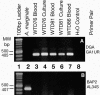
We used tick cell culture to isolate a bacterium previously referred to as the "white-tailed deer (WTD) agent" from two captive fawns inoculated with blood from wild WTD (Odocoileus virginianus). Buffy coat cells were added to ISE6 tick cell cultures and incubated at 34 degrees C, and 8 days later, Anaplasma-like inclusions were demonstrated in Giemsa-stained culture samples. The microbes became established and could be continuously passaged in tick cells. The identity of a culture isolate designated WTD76 was verified as the WTD agent by using specific PCR primers and by DNA sequencing. Comparison with sequences available in GenBank indicated that the isolate was most closely related first to Anaplasma platys and second to Anaplasma phagocytophilum, supporting its placement in the genus Anaplasma. Transmission electron microscopy of this Anaplasma sp. organism in tick cell cultures revealed large inclusions filled with pleomorphic and rod-shaped bacteria. Tick cells infected with the Anaplasma sp. organism were used to successfully infect a naive deer, thereby proving the infectivity of the isolate for deer.
Figures



References
-
- Anderson, B. E., C. E. Greene, D. C. Jones, and J. E. Dawson. 1992. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int. J. Syst. Bacteriol. 42:299-302. - PubMed
-
- Anziani, O. S., S. A. Ewing, and R. W. Barker. 1990. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res. 51:929-931. - PubMed
-
- Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

