Protean agonism at histamine H3 receptors in vitro and in vivo
- PMID: 12960366
- PMCID: PMC196931
- DOI: 10.1073/pnas.1932276100
Protean agonism at histamine H3 receptors in vitro and in vivo
Abstract
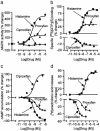
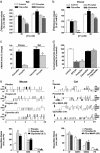
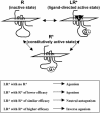
G protein-coupled receptors (GPCRs) are allosteric proteins that adopt inactive (R) and active (R*) conformations in equilibrium. R* is promoted by agonists or occurs spontaneously, leading to constitutive activity of the receptor. Conversely, inverse agonists promote R and decrease constitutive activity. The existence of another pharmacological entity, referred to as "protean" agonists (after Proteus, the Greek god who could change shape), was assumed on theoretical grounds. It was predicted from the existence of constitutive activity that a same ligand of this class could act either as an agonist or an inverse agonist at the same GPCR. Here, we show that proxyfan, a high-affinity histamine H3-receptor ligand, acts as a protean agonist at recombinant H3 receptors expressed in the same Chinese hamster ovary cells. In support of the physiological relevance of the process, we show that proxyfan also behaves as a protean agonist at native H3 receptors known to display constitutive activity. On neurochemical and behavioral responses in rodents and cats, proxyfan displays a spectrum of activity ranging from full agonism to full inverse agonism. Thus, protean agonism demonstrates the existence of ligand-directed active states LR* different from, and competing with, constitutively active states R* of GPCRs, and defines a pharmacological entity with important therapeutic implications.
Figures
References
-
- Samama, P., Cotecchia, S., Costa, T. & Lefkowitz, R. J. (1993) J. Biol. Chem. 268, 4625-4636. - PubMed
-
- Leff, P. (1995) Trends Pharmacol. Sci. 16, 89-97. - PubMed
-
- Weiss, J. M., Morgan, P. H., Lutz, M. W. & Kenakin, T. P. (1996) J. Theor. Biol. 181, 381-397. - PubMed
-
- Lefkowitz, R. J. Cotecchia, S., Samama, P. & Costa, T. (1993) Trends Pharmacol. Sci. 14, 303-307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

