Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases
- PMID: 12960370
- PMCID: PMC196862
- DOI: 10.1073/pnas.1932854100
Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases
Abstract
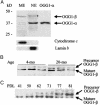
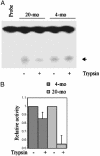
The mitochondria are the major source of chronic oxidative stress, which has been implicated in the aging process. Along with other cellular changes, aged cells accumulate mutations in both their nuclear and mitochondrial genomes, and they contain increased amounts of oxidatively damaged mutagenic bases such as 7,8-dihydro-8-oxoguanine, suggesting age-dependent inhibition of its repair. Surprisingly, the level and activity of 8-oxoguanine-DNA glycosylase (OGG1), responsible for repair of 7,8-dihydro-8-oxoguanine, was found to be higher in the liver mitochondrial extract from old rodents than in that from young ones. We addressed this paradox by analyzing OGG1 in the mitochondria of young vs. old mouse livers, as well as in replicating vs. presenescent human fibroblasts. We show here that although the total OGG1 activity is higher in old mitochondria, a large fraction of the enzyme is stuck to the membrane in the precursor form, which could not be translocated to and processed in the mitochondrial matrix. A nearly identical phenomenon was observed with the mitochondrial uracil-DNA glycosylase responsible for repair of mutagenic uracil. These results indicate an age-dependent decline in the mitochondrial import of proteins needed for DNA repair and possibly for other functions.
Figures



References
-
- Beckman, K. B. & Ames, B. N. (1998) Physiol. Rev. 78, 547–581. - PubMed
-
- Cortopassi, G. A. & Wong, A. (1999) Biochim. Biophys. Acta 1410, 183–193. - PubMed
-
- Sohal, R. S., Mockett, R. J. & Orr, W. C. (2002) Free Radical Biol. Med. 33, 575–586. - PubMed
-
- Kowald, A. & Kirkwood, T. B. (1993) Mutat. Res. 295, 93–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

