Coordination of central odor representations through transient, non-oscillatory synchronization of glomerular output neurons
- PMID: 12960372
- PMCID: PMC196929
- DOI: 10.1073/pnas.1934001100
Coordination of central odor representations through transient, non-oscillatory synchronization of glomerular output neurons
Abstract
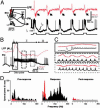
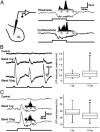
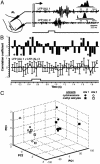
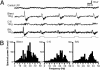
At the first stage of processing in the olfactory pathway, the patterns of glomerular activity evoked by different scents are both temporally and spatially dynamic. In the antennal lobe (AL) of some insects, coherent firing of AL projection neurons (PNs) can be phase-locked to network oscillations, and it has been proposed that oscillatory synchronization of PN activity may encode the chemical identity of the olfactory stimulus. It remains unclear, however, how the brain uses this time-constrained mechanism to encode chemical identity when the stimulus itself is unpredictably dynamic. In the olfactory pathway of the moth Manduca sexta,we find that different odorants evoke gamma-band oscillations in the AL and the mushroom body (a higher-order network that receives input from the AL), but oscillations within or between these two processing stages are not temporally coherent. Moreover, the timing of action potential firing in PNs is not phase-locked to oscillations in either the AL or mushroom body, and the correlation between PN synchrony and field oscillations remains low before, during, and after olfactory stimulation. These results demonstrate that olfactory circuits in the moth are specialized to preserve time-varying signals in the insect's olfactory space, and that stimulus dynamics rather than intrinsic oscillations modulate the uniquely coordinated pattern of PN synchronization evoked by each olfactory stimulus. We propose that non-oscillatory synchronization provides an adaptive mechanism by which PN ensembles can encode stimulus identity while concurrently monitoring the unpredictable dynamics in the olfactory signal that typically occur under natural stimulus conditions.
Figures
References
-
- Gelperin, A. (1999) J. Exp. Biol. 202, 1855–1864. - PubMed
-
- Mori, K., Nagao, H. & Yoshihara, Y. (1999) Science 286, 711–715. - PubMed
-
- Laurent, G. (1999) Science 286, 723–728. - PubMed
-
- Christensen, T. A. & White, J. (2000) in The Neurobiology of Taste and Smell, eds. Finger, T. E., Silver, W. L. & Restrepo, D. (Wiley, New York), pp. 201–232.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

