(+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata)
- PMID: 12960376
- PMCID: PMC196857
- DOI: 10.1073/pnas.1934562100
(+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata)
Abstract
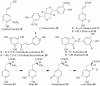
An enantio-specific polyphenol oxidase (PPO) was purified approximately 1,700-fold to apparent homogeneity from the creosote bush (Larrea tridentata), and its encoding gene was cloned. The posttranslationally processed PPO ( approximately 43 kDa) has a central role in the biosynthesis of the creosote bush 8-8' linked lignans, whose representatives, such as nordihydroguaiaretic acid and its congeners, have potent antiviral, anticancer, and antioxidant properties. The PPO primarily engenders the enantio-specific conversion of (+)-larreatricin into (+)-3'-hydroxylarreatricin, with the regiochemistry of catalysis being unambiguously established by different NMR spectroscopic analyses; the corresponding (-)-enantiomer did not serve as a substrate. This enantio-specificity for a PPO, a representative of a widespread class of enzymes, provides additional insight into their actual physiological roles that hitherto have been difficult to determine.
Figures
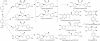


Similar articles
-
Synthesis and chiral HPLC analysis of the dibenzyltetrahydrofuran lignans, larreatricins, 8'-epi-larreatricins, 3,3'-didemethoxyverrucosins and meso-3,3'-didemethoxynectandrin B in the creosote bush (Larrea tridentata): evidence for regiospecific control of coupling.Org Biomol Chem. 2003 Jul 7;1(13):2307-13. doi: 10.1039/b302632a. Org Biomol Chem. 2003. PMID: 12945702
-
A pinoresinol-lariciresinol reductase homologue from the creosote bush (Larrea tridentata) catalyzes the efficient in vitro conversion of p-coumaryl/coniferyl alcohol esters into the allylphenols chavicol/eugenol, but not the propenylphenols p-anol/isoeugenol.Arch Biochem Biophys. 2007 Sep 1;465(1):209-18. doi: 10.1016/j.abb.2007.06.002. Epub 2007 Jun 9. Arch Biochem Biophys. 2007. PMID: 17624297
-
Allyl/propenyl phenol synthases from the creosote bush and engineering production of specialty/commodity chemicals, eugenol/isoeugenol, in Escherichia coli.Arch Biochem Biophys. 2014 Jan 1;541:37-46. doi: 10.1016/j.abb.2013.10.019. Epub 2013 Nov 1. Arch Biochem Biophys. 2014. PMID: 24189289
-
Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid.J Ethnopharmacol. 2005 Apr 26;98(3):231-9. doi: 10.1016/j.jep.2005.02.002. J Ethnopharmacol. 2005. PMID: 15814253 Review.
-
Creosote bush lignans for human disease treatment and prevention: Perspectives on combination therapy.J Tradit Complement Med. 2015 Mar 12;5(3):119-26. doi: 10.1016/j.jtcme.2014.11.024. eCollection 2015 Jul. J Tradit Complement Med. 2015. PMID: 26151022 Free PMC article. Review.
Cited by
-
The PPO family in Nicotiana tabacum is an important regulator to participate in pollination.BMC Plant Biol. 2024 Feb 9;24(1):102. doi: 10.1186/s12870-024-04769-3. BMC Plant Biol. 2024. PMID: 38331761 Free PMC article.
-
Plant Copper Metalloenzymes As Prospects for New Metabolism Involving Aromatic Compounds.Front Plant Sci. 2021 Nov 29;12:692108. doi: 10.3389/fpls.2021.692108. eCollection 2021. Front Plant Sci. 2021. PMID: 34925392 Free PMC article. Review.
-
Beyond brown: polyphenol oxidases as enzymes of plant specialized metabolism.Front Plant Sci. 2015 Jan 14;5:783. doi: 10.3389/fpls.2014.00783. eCollection 2014. Front Plant Sci. 2015. PMID: 25642234 Free PMC article. Review.
-
Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut.Plant Physiol. 2014 Mar;164(3):1191-203. doi: 10.1104/pp.113.228593. Epub 2014 Jan 21. Plant Physiol. 2014. PMID: 24449710 Free PMC article.
-
Larrea tridentata: A novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri.PLoS Negl Trop Dis. 2017 Aug 9;11(8):e0005832. doi: 10.1371/journal.pntd.0005832. eCollection 2017 Aug. PLoS Negl Trop Dis. 2017. PMID: 28793307 Free PMC article.
References
-
- Train, P., Henrichs, J. R. & Archer, W. A. (1941) Medicinal Uses of Plants by Indian Tribes of Nevada (U.S. Department of Agriculture, Washington, DC).
-
- Waller, C. W. & Gisvold, O. (1945) J. Am. Pharm. Assoc. 34, 78–81.
-
- Timmermann, B. N. (1977) in Creosote Bush: Biology and Chemistry of Larrea in New World Deserts, eds. Mabry, T. J., Hunziker, J. H. & DiFeo, D. R., Jr. (Dowden, Hutchinson & Ross, Stroudsburg, PA), pp. 252–276.
-
- Lundberg, W. O., Halvorson, H. O. & Burr, G. O. (1944) Oil Soap 21, 33–35.
-
- Oliveto, E. P. (1972) Chem. Industry 677–679.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

