Ionizing radiation induces heritable disruption of epithelial cell interactions
- PMID: 12960393
- PMCID: PMC196872
- DOI: 10.1073/pnas.1832185100
Ionizing radiation induces heritable disruption of epithelial cell interactions
Abstract
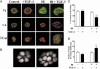
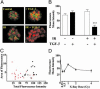
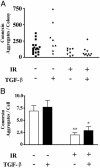
Ionizing radiation (IR) is a known human breast carcinogen. Although the mutagenic capacity of IR is widely acknowledged as the basis for its action as a carcinogen, we and others have shown that IR can also induce growth factors and extracellular matrix remodeling. As a consequence, we have proposed that an additional factor contributing to IR carcinogenesis is the potential disruption of critical constraints that are imposed by normal cell interactions. To test this hypothesis, we asked whether IR affected the ability of nonmalignant human mammary epithelial cells (HMEC) to undergo tissue-specific morphogenesis in culture by using confocal microscopy and imaging bioinformatics. We found that irradiated single HMEC gave rise to colonies exhibiting decreased localization of E-cadherin, beta-catenin, and connexin-43, proteins necessary for the establishment of polarity and communication. Severely compromised acinar organization was manifested by the majority of irradiated HMEC progeny as quantified by image analysis. Disrupted cell-cell communication, aberrant cell-extracellular matrix interactions, and loss of tissue-specific architecture observed in the daughters of irradiated HMEC are characteristic of neoplastic progression. These data point to a heritable, nonmutational mechanism whereby IR compromises cell polarity and multicellular organization.
Figures

References
-
- Mattsson, A., Ruden, B.-I., Wilking, N. & Rutqvist, L. E. (1993) J. Natl. Cancer Inst. 85, 1679–1685. - PubMed
-
- Mauch, P. (1995) Int. J. Radiat. Oncol. Biol. Phys. 33, 959–960. - PubMed
-
- Davis, F. G., Boice, J. D., Hrubec, Z. & Monson, R. R. (1989) Cancer Res. 49, 6130–6136. - PubMed
-
- Tokunaga, M., Land, C. E., Yamamoto, T., Asano, M., Tokuoka, S., Ezaki, H. & Nishimori, I. (1987) Radiat. Res. 112, 243–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

